Optimizing SCNT Embryo Development: A Comprehensive Guide to the JNJ-7706621 Treatment Protocol
Somatic cell nuclear transfer (SCNT) is a pivotal technology for animal cloning and biomedical research, yet its efficiency is hampered by low developmental rates and frequent embryonic abnormalities.
Optimizing SCNT Embryo Development: A Comprehensive Guide to the JNJ-7706621 Treatment Protocol
Abstract
Somatic cell nuclear transfer (SCNT) is a pivotal technology for animal cloning and biomedical research, yet its efficiency is hampered by low developmental rates and frequent embryonic abnormalities. This article provides a detailed examination of a novel pharmacological approach using JNJ-7706621, a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and Aurora kinases, to significantly enhance SCNT outcomes. We explore the foundational science behind SCNT reprogramming barriers, present a step-by-step methodology for JNJ-7706621 application based on recent peer-reviewed studies, and offer troubleshooting strategies for protocol optimization. Comparative analyses validate its superiority over traditional methods like cytochalasin B, demonstrating marked improvements in blastocyst formation, cell number, cytoskeletal integrity, and, crucially, live birth rates in mouse models. This resource is tailored for researchers and drug development professionals seeking to refine SCNT protocols for more reliable and efficient results in regenerative medicine and biotechnology.
Understanding SCNT Reprogramming Barriers and the Rationale for JNJ-7706621
Somatic cell nuclear transfer (SCNT) is a revolutionary assisted reproduction technology that enables the reprogramming of terminally differentiated somatic cells into a totipotent state by transferring their nuclei into enucleated oocytes [1] [2]. Since the landmark birth of Dolly the sheep in 1996, SCNT has successfully produced cloned offspring in more than 20 mammalian species, including mice, cattle, pigs, and non-human primates [2] [3]. This technology holds tremendous potential for multiplying genetically valuable animals, wildlife conservation, generating genetically modified animal models, and producing therapeutic proteins through mammary gland bioreactors [1] [2]. Despite these significant achievements, the practical application of SCNT remains severely limited by persistently low cloning efficiency, which typically ranges between 1% and 5% across most species [4] [5].
The primary bottleneck in SCNT efficiency stems from incomplete epigenetic reprogramming of the donor somatic cell nucleus [2] [4] [5]. Epigenetic modifications—including DNA methylation, histone modifications, genomic imprinting, and X-chromosome inactivation—represent heritable changes in gene expression potential that occur without alterations to the underlying DNA sequence [2]. During normal embryonic development, these epigenetic marks are systematically erased and reestablished to enable proper totipotent programming. However, in SCNT embryos, the epigenetic memory of the donor somatic cell creates substantial barriers that impede this reprogramming process, leading to abnormal gene expression, developmental arrest, and frequently, cloned offspring with abnormalities [1] [4] [5].
Fundamental Epigenetic Barriers in SCNT
The epigenetic barriers in SCNT can be broadly categorized into two classes based on their temporal manifestation during embryonic development: pre-implantation and post-implantation defects.
Pre-implantation Epigenetic Barriers
Pre-implantation development encompasses the critical period from embryo reconstruction to blastocyst formation, during which two pivotal events occur: zygotic genome activation (ZGA) and the establishment of trophectoderm (TE) and inner cell mass (ICM) lineages [4]. In SCNT embryos, this phase is marked by several profound epigenetic obstacles that frequently lead to developmental arrest.
Abnormal Histone Modifications: SCNT embryos consistently demonstrate aberrant patterns of histone modifications, particularly excessive H3K9me3 deposition, which creates a repressive chromatin state that blocks access to essential embryonic genes during ZGA [6] [4]. Additionally, abnormal H3K4me3 methylation and insufficient histone acetylation further contribute to defective reprogramming [6] [7]. These aberrant histone marks silence critical developmental genes, preventing the proper activation of the embryonic transcriptional program [4].
Defective DNA Methylation Reprogramming: The somatic donor cell genome carries highly methylated DNA patterns that must be extensively demethylated during reprogramming [1] [2]. However, SCNT embryos exhibit delayed and incomplete DNA demethylation, leading to retained methylation at gene promoters that should be activated during pre-implantation development [2]. This results in the persistent silencing of pluripotency-associated genes and other factors crucial for embryonic development [1].
Zygotic Genome Activation Failure: The culmination of these epigenetic defects frequently manifests as incomplete ZGA, where SCNT embryos fail to properly transition from maternal to embryonic gene control [4] [8]. This failure is particularly evident in the insufficient upregulation of ZGA-related genes such as ZSCAN4, UBTFL1, and SUPT4H1, which are essential for subsequent embryonic development [7].
Post-implantation Epigenetic Barriers
Even when SCNT embryos successfully reach the blastocyst stage, they face additional epigenetic challenges that compromise post-implantation development and full-term viability.
Loss of H3K27me3-Mediated Imprinting: A critical post-implantation barrier involves the aberrant loss of non-canonical imprinting regulated by H3K27me3 [6]. In normal development, H3K27me3 maintains the monoallelic expression of specific genes in extraembryonic lineages. SCNT embryos frequently fail to maintain these imprinted marks, leading to biallelic expression of genes such as Sfmbt2, Jade1, Gab1, and Smoc1, which causes severe placental abnormalities and subsequent fetal loss [6] [4].
X-Chromosome Inactivation Defects: Female SCNT embryos often exhibit ectopic Xist expression, the master regulator of X-chromosome inactivation [4]. This dysregulation leads to abnormal silencing of X-linked genes, creating an imbalance in gene dosage that compromises embryonic viability and contributes to developmental abnormalities in cloned conceptuses [4].
Table 1: Major Epigenetic Barriers in SCNT Embryos
| Developmental Stage | Epigenetic Barrier | Molecular Consequence | Developmental Outcome |
|---|---|---|---|
| Pre-implantation | Aberrant H3K9me3 deposition | Silencing of embryonic genes | Arrest at ZGA stage |
| Pre-implantation | Abnormal H3K4me3 patterns | Disrupted transcriptional activation | Failed lineage specification |
| Pre-implantation | Defective histone acetylation | Chromatin compaction | Reduced reprogramming efficiency |
| Pre-implantation | Persistent DNA methylation | Gene silencing | Blastocyst formation failure |
| Post-implantation | Loss of H3K27me3 imprinting | Biallelic expression of imprinted genes | Placental abnormalities |
| Post-implantation | Ectopic Xist expression | Abnormal X-chromosome silencing | Fetal loss and abnormalities |
Quantitative Assessment of SCNT Efficiency
The low efficiency of SCNT technology is evident across multiple developmental stages. The following table summarizes typical success rates for SCNT embryos compared with normal embryonic development, highlighting the profound efficiency gap that exists throughout the developmental continuum.
Table 2: Developmental Efficiency Comparison Between SCNT and Normal Embryos
| Development Stage | SCNT Embryos | Normal Embryos | Key Contributing Factors |
|---|---|---|---|
| Blastocyst Formation | 30-40% [8] | 70-80% [8] | Incomplete epigenetic reprogramming [1] |
| Post-implantation Development | 10-20% [6] | 50-60% | Loss of H3K27me3 imprinting [6] |
| Full-term Development | 1-5% [4] [5] | 40-50% | Cumulative epigenetic barriers [6] |
| Cloned Offspring Viability | High abnormality rate [5] | Normal development | Placental dysfunction [4] |
Recent research has demonstrated promising strategies for overcoming these efficiency limitations. A 2025 study reported that combining Kdm4d and Kdm5b overexpression with trichostatin A (TSA) treatment, alongside tetraploid complementation, achieved approximately 30% full-term development efficiency in mouse SCNT embryos—representing the highest cloning efficiency reported in mammals to date [6]. This breakthrough highlights the potential of targeted epigenetic interventions to substantially improve SCNT outcomes.
JNJ-7706621 Treatment Protocol for SCNT Improvement
Background and Mechanism of Action
JNJ-7706621 is a cyclin-dependent kinase (CDK) inhibitor that has demonstrated significant potential for improving SCNT outcomes through its effect on M-phase promoting factor (MPF) regulation [9]. MPF, a complex of cyclin B and CDK1, plays a crucial role in controlling the cell cycle transition from M-phase to interphase. In SCNT embryos, properly modulating MPF activity following activation is essential for successful nuclear reprogramming and subsequent embryonic development [9].
The compound functions through dual mechanisms: it significantly elevates Tyr15 phosphorylation of CDK1 while simultaneously reducing Thr161 phosphorylation of the same protein [9]. This combined effect results in substantial suppression of CDK1 activity and a consequent reduction in overall MPF levels, creating a more favorable environment for nuclear envelope breakdown and premature chromosome condensation (PCC)—critical early events in nuclear reprogramming following SCNT [9] [3].
Detailed Experimental Protocol
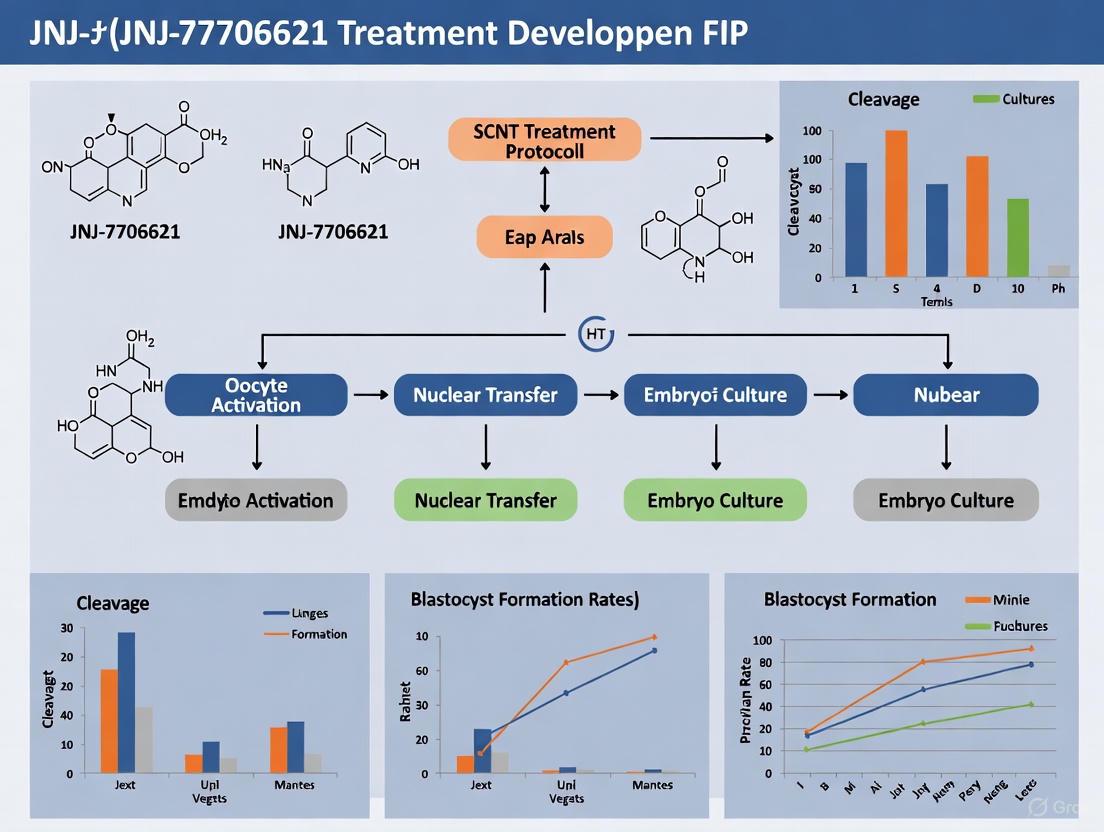
The following workflow diagram illustrates the key steps in implementing JNJ-7706621 treatment during SCNT procedures:
Reagent Preparation
- JNJ-7706621 Stock Solution: Prepare a 10 mM stock solution by dissolving JNJ-7706621 in high-quality DMSO. Aliquot and store at -20°C protected from light.
- Working Solution: Dilute the stock solution in pre-equilibrated embryo culture medium to achieve a final concentration of 10 µM JNJ-7706621. Ensure the final DMSO concentration does not exceed 0.1% (v/v).
- Control Solutions: Prepare control treatments including (1) culture medium with equivalent DMSO concentration (vehicle control), and (2) 5 µg/mL cytochalasin B for comparative assessment [9].
Treatment Procedure
- SCNT Embryo Reconstruction: Perform standard SCNT procedures using donor somatic cells and enucleated MII oocytes appropriate for your species of interest [1] [3].
- Artificial Activation: Activate reconstructed embryos using species-appropriate activation protocols (e.g., strontium chloride treatment for mouse embryos) [3].
- JNJ-7706621 Treatment: Immediately post-activation, transfer the embryos into culture medium containing 10 µM JNJ-7706621 [9].
- Incubation Duration: Maintain embryos in JNJ-7706621-containing medium for precisely 4 hours at standard culture conditions (37°C, 5% CO₂ in air) [9].
- Wash and Culture: Thoroughly wash embryos three times in fresh culture medium to completely remove JNJ-7706621, then transfer to standard in vitro culture (IVC) medium for continued development.
- Developmental Assessment: Monitor and record cleavage rates, blastocyst formation, and quality parameters (cell number, apoptosis) throughout the culture period [9].
Outcome Assessment and Validation
The efficacy of JNJ-7706621 treatment should be evaluated through multiple developmental and molecular parameters:
- Developmental Rates: Treated SCNT embryos demonstrate significantly improved blastocyst formation rates compared to both vehicle control and cytochalasin B-treated embryos [9].
- Molecular Analysis: Confirm treatment effectiveness by assessing MPF activity through measurement of CDK1 phosphorylation status at Tyr15 and Thr161 residues, with successful treatment showing elevated Tyr15 phosphorylation and reduced Thr161 phosphorylation [9].
- Quality Assessment: Evaluate blastocyst quality through differential staining of inner cell mass (ICM) and trophectoderm (TE) cells, with successful treatment typically resulting in increased total cell numbers and improved ICM:TE ratio [9].
Integrated Epigenetic Manipulation Strategies
While JNJ-7706621 primarily targets cell cycle regulation, comprehensive improvement of SCNT outcomes often requires combining multiple approaches to address the diverse epigenetic barriers simultaneously. The following diagram illustrates the key epigenetic barriers and corresponding intervention strategies:
Combinatorial Epigenetic Modulation
Recent advances demonstrate that the most significant improvements in SCNT efficiency come from strategically combining multiple epigenetic interventions:
Histone Modification Correction: Simultaneous overexpression of Kdm4d and Kdm5b mRNA in SCNT embryos effectively removes aberrant H3K9me3 and H3K4me3 marks, respectively, dramatically improving pre-implantation development [6]. When combined with histone deacetylase inhibitors like trichostatin A (TSA), this approach creates a more permissive chromatin state that facilitates essential embryonic gene activation during ZGA [6].
Tetraploid Complementation: For addressing post-implantation defects, particularly placental abnormalities stemming from loss of H3K27me3-mediated imprinting, tetraploid complementation has proven highly effective [6]. This technique involves aggregating SCNT-derived diploid embryos with tetraploid host embryos, which preferentially contribute to the extraembryonic lineages while the fetus develops exclusively from the SCNT cells, thereby bypassing placental defects [6].
Antioxidant Supplementation: Emerging evidence indicates that oxidative stress compounds epigenetic reprogramming defects in SCNT embryos. Supplementation with potent antioxidants such as lycopene (0.2 µM) during in vitro culture has been shown to reduce reactive oxygen species, improve mitochondrial membrane potential, enhance autophagy, and promote more favorable epigenetic patterns including reduced H3K9me3 and DNA methylation levels [7].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for SCNT Epigenetic Manipulation
| Reagent | Category | Function in SCNT | Typical Concentration |
|---|---|---|---|
| JNJ-7706621 | CDK Inhibitor | Suppresses MPF activity, improves reprogramming | 10 µM for 4 hours [9] |
| Trichostatin A (TSA) | HDAC Inhibitor | Increases histone acetylation, opens chromatin | 50 nM for 8-12 hours [6] |
| Kdm4d mRNA | Histone Demethylase | Removes H3K9me3 barriers | 500-1000 ng/µL mRNA injection [6] |
| Kdm5b mRNA | Histone Demethylase | Corrects H3K4me3 abnormalities | 500-1000 ng/µL mRNA injection [6] |
| Lycopene | Antioxidant | Reduces ROS, improves epigenetic reprogramming | 0.2 µM during IVC [7] |
| 5-aza-2'-deoxycytidine | DNMT Inhibitor | Reduces DNA methylation | 0.5-1.0 µM for 6-8 hours [1] |
| Linaprazan mesylate | Linaprazan mesylate, CAS:855998-67-3, MF:C22H30N4O5S, MW:462.6 g/mol | Chemical Reagent | Bench Chemicals |
| Metipranolol Hydrochloride | Metipranolol Hydrochloride, CAS:36592-77-5, MF:C17H28ClNO4, MW:345.9 g/mol | Chemical Reagent | Bench Chemicals |
The fundamental challenges of SCNT—epigenetic barriers and low efficiency—remain significant obstacles to the widespread application of this powerful technology. However, recent advances in understanding the molecular mechanisms underlying epigenetic reprogramming have led to the development of targeted intervention strategies that show remarkable promise.
The JNJ-7706621 treatment protocol represents a valuable approach for improving SCNT outcomes through its effect on cell cycle regulation and MPF activity. When integrated with complementary strategies addressing histone modifications, DNA methylation, and oxidative stress, substantial improvements in cloning efficiency can be achieved. The recent report of approximately 30% full-term development efficiency in mouse SCNT embryos through combined epigenetic modulation demonstrates the potential for overcoming the historical limitations of this technology [6].
Future research directions should focus on refining the timing and dosage of these epigenetic interventions, developing species-specific optimization protocols, and exploring novel small molecule approaches that might further enhance reprogramming efficiency. As our understanding of epigenetic regulation continues to deepen, the gap between current SCNT efficiency and practical application will undoubtedly narrow, unlocking the full potential of this revolutionary technology for both basic research and translational medicine.
Somatic Cell Nuclear Transfer (SCNT) is a pivotal technique in reproductive biology and regenerative medicine, enabling the reprogramming of a somatic cell nucleus to a totipotent state. Despite its potential, SCNT efficiency remains critically low, primarily due to inadequate epigenetic reprogramming and aberrant cell cycle regulation [10]. The core regulators of the cell cycle, Cyclin-Dependent Kinase 1 (CDK1) and its complex with Cyclin B, known as M-Phase Promoting Factor (MPF), are instrumental in orchestrating nuclear envelope breakdown, chromosome condensation, and spindle assembly—processes fundamental to successful SCNT [11] [12]. This application note details a specialized treatment protocol utilizing JNJ-7706621, a selective inhibitor of CDK1 and Aurora kinases, to modulate MPF activity and enhance the developmental competence of SCNT embryos. We provide consolidated quantitative data, standardized experimental workflows, and essential reagent solutions to support implementation of this protocol in research settings.
Quantitative Efficacy of JNJ-7706621 in SCNT Embryo Development
Treatment with JNJ-7706621 consistently and significantly enhances pre- and post-implantation development of SCNT embryos across multiple species. The tables below summarize key quantitative findings from published studies.
Table 1: Pre-implantation Development of SCNT Embryos Treated with JNJ-7706621
| Species | Treatment | Blastocyst Rate (%) | Total Cell Number | Inner Cell Mass (ICM) | Trophectoderm (TE) |
|---|---|---|---|---|---|
| Mouse [13] | Cytochalasin B (CB) | 39.9 ± 6.4 | 52.7 ± 3.6 | 10.4 ± 0.7 | 42.3 ± 3.3 |
| Mouse [13] | JNJ-7706621 (10 µM) | 61.4 ± 4.4 | 70.7 ± 2.9 | 15.4 ± 1.1 | 55.3 ± 2.5 |
| Porcine [9] | Cytochalasin B (CB) | *Reported as significantly lower | - | - | - |
| Porcine [9] | JNJ-7706621 (10 µM) | *Reported as significantly higher | - | - | - |
*Note: The porcine study [9] confirmed significantly higher blastocyst rates with JNJ but did not report specific mean values with standard errors for all parameters.
Table 2: Post-implantation and Full-Term Development of Mouse SCNT Embryos
| Development Parameter | Cytochalasin B (CB) | JNJ-7706621 (10 µM) |
|---|---|---|
| Implantation Rate (%) | 50.8 ± 3.7 | 68.3 ± 4.3 |
| Live Birth Rate (%) | 2.4 ± 2.4 | 10.9 ± 2.8 |
JNJ-7706621 Treatment Protocol for SCNT Embryos
Principle
JNJ-7706621 is a dual-specificity inhibitor targeting CDK1 and Aurora kinases [14]. Its application during SCNT embryo activation suppresses CDK1 activity and reduces MPF levels, which promotes proper chromosome segregation, reduces cytoskeletal abnormalities, and enhances epigenetic reprogramming [13] [9].
Materials and Reagents
- JNJ-7706621 (Cat. No. SML0571): Prepare a 10 mM stock solution in DMSO. Aliquot and store at -20°C.
- Culture Medium: Appropriate embryo culture medium (e.g., KSOM, PZM).
- Activation Solution: Depending on protocol, may contain SrClâ‚‚ for parthenogenetic activation or specific reagents for SCNT.
Step-by-Step Procedure
- SCNT Embryo Production: Perform standard SCNT procedures, including oocyte enucleation, donor cell fusion, and artificial activation [10].
- Post-Activation Treatment: Immediately after activation, transfer SCNT embryos into culture medium supplemented with 10 µM JNJ-7706621.
- Incubation: Culture embryos in the JNJ-7706621-containing medium for 4 hours at 37°C under 5% CO₂.
- Washing and Continued Culture: After treatment, wash embryos thoroughly with fresh culture medium three times to remove the inhibitor. Transfer embryos to fresh culture medium and continue culture under standard conditions until blastocyst stage or embryo transfer.
Molecular Mechanism of JNJ-7706621 Action
JNJ-7706621 enhances SCNT efficiency by directly targeting the CDK1/MPF core regulatory axis. The diagram below illustrates the signaling pathway and the specific points of JNJ-7706621 intervention.
The molecular mechanism involves:
- CDK1 Inhibition: JNJ-7706621 directly inhibits CDK1 kinase activity, which is indispensable for mitotic entry and progression. This leads to a reduction in MPF activity, facilitating proper nuclear remodeling [11] [15].
- Aurora Kinase Inhibition: Concurrent inhibition of Aurora kinases promotes correct chromosome alignment and segregation, mitigating aneuploidy [13].
- Epigenetic Modulation: High CDK1 activity in pluripotent cells phosphorylates epigenetic regulators, including the H3K79 methyltransferase Dot1l. By modulating CDK1 activity, JNJ-7706621 influences the global epigenetic landscape, promoting a state conducive to reprogramming [16].
- Cytoskeletal Stabilization: Treatment significantly reduces aberrant F-actin and tubulin structures, leading to improved spindle morphology and decreased blastomere fragmentation [13].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for SCNT and Cell Cycle Research
| Reagent / Assay | Specific Example / Catalog Number | Primary Function in Protocol |
|---|---|---|
| CDK1/Aurora Kinase Inhibitor | JNJ-7706621 (e.g., Sigma SML0571) | Core therapeutic: Suppresses CDK1 & Aurora kinase activity to optimize reprogramming. |
| Microtubule Staining | Anti-α-Tubulin Antibody | Visualizes spindle morphology and integrity post-treatment. |
| DNA Damage Assay | γH2AX Immunostaining | Quantifies DNA double-strand breaks in early embryos. |
| Cell Death Detection TUNEL Assay | TUNEL Assay Kit (e.g., Roche) | Apoptosis detection in blastocysts; assesses embryonic health. |
| MPF Activity Assay | CDK1 (pThr161) Antibody / H1 Kinase Assay | Direct measurement of MPF activity levels in oocytes/embryos. |
| Live-Cell Imaging | FUCCI (Fluorescent Ubiquitination-based Cell Cycle Indicator) | Real-time visualization of cell cycle dynamics in live embryos. |
| Miocamycin | Miocamycin, CAS:55881-07-7, MF:C45H71NO17, MW:898.0 g/mol | Chemical Reagent |
| Misonidazole | Misonidazole, CAS:13551-87-6, MF:C7H11N3O4, MW:201.18 g/mol | Chemical Reagent |
Modulation of the CDK1/MPF axis through post-activation treatment with JNJ-7706621 presents a robust and reproducible method to significantly enhance the developmental competence of SCNT embryos. The protocol outlined herein provides a clear framework for leveraging this small molecule inhibitor to overcome key bottlenecks in SCNT, including cytoskeletal defects, chromosomal instability, and inadequate epigenetic reprogramming. By integrating this targeted pharmacological approach with refined SCNT techniques, researchers can achieve substantial improvements in both pre- and post-implantation development, accelerating progress in therapeutic cloning and regenerative medicine applications.
JNJ-7706621 is a novel synthetic organic compound identified as a potent, ATP-competitive dual inhibitor of cyclin-dependent kinases (CDKs) and Aurora kinases (AURKs). Its primary mechanism of action involves targeted inhibition of key cell cycle regulators: it shows highest potency against CDK1 (ICâ‚…â‚€ = 9 nM) and CDK2 (ICâ‚…â‚€ = 3-4 nM) in cell-free assays, while also strongly inhibiting Aurora A (ICâ‚…â‚€ = 11 nM) and Aurora B (ICâ‚…â‚€ = 15 nM) [17] [18]. This unique inhibition profile enables JNJ-7706621 to simultaneously target multiple phases of cell cycle progression, inducing Gâ‚ delay and Gâ‚‚-M cell cycle arrest in human cancer cells, followed by activation of apoptosis and reduced colony formation independent of p53, retinoblastoma, or P-glycoprotein status [18].
Table 1: Kinase Inhibition Profile of JNJ-7706621
| Target Kinase | ICâ‚…â‚€ Value (nM) | Cellular Function |
|---|---|---|
| CDK1/Cyclin B | 9 | Gâ‚‚/M transition regulator |
| CDK2/Cyclin E | 3 | Gâ‚/S transition regulator |
| CDK2/Cyclin A | 4 | S phase progression |
| Aurora A | 11 | Mitotic spindle assembly |
| Aurora B | 15 | Chromosome segregation |
| CDK5-p35 | 0.2* (% activity remaining at 0.5µM) | Neuronal differentiation |
Note: *Value represents % activity remaining at 0.5µM concentration [19]
Beyond its established anticancer properties, recent investigations have revealed the significant potential of JNJ-7706621 as a strategic intervention to enhance the developmental competence of somatic cell nuclear transfer (SCNT) embryos. By modulating the critical CDK1-mediated pathways that govern early embryonic development, JNJ-7706621 has demonstrated remarkable efficacy in improving preimplantation development and full-term success rates in mammalian cloning research [13] [9].
Mechanism of Action: Coordinated Cell Cycle Regulation
The therapeutic efficacy of JNJ-7706621 stems from its coordinated inhibition of complementary cell cycle regulatory pathways. CDK1, when complexed with cyclin B, forms maturation-promoting factor (MPF), the primary driver of the Gâ‚‚/M transition. Concurrently, Aurora kinases regulate crucial mitotic processes including spindle assembly, chromosome segregation, and cytokinesis [20] [21].
In SCNT embryos, the balanced activity of these kinases is critical for proper nuclear reprogramming and embryonic development. JNJ-7706621 treatment specifically suppresses CDK1 activity and concomitantly reduces MPF levels, creating a favorable environment for nuclear remodeling [9]. Simultaneous Aurora kinase inhibition prevents chromosomal mis-segregation and mitotic errors that commonly compromise SCNT embryo viability [13].
Figure 1: Mechanism of JNJ-7706621 Action in SCNT Embryos. The diagram illustrates how dual inhibition of CDK1 and Aurora kinases coordinates improved embryonic development.
The interconnected CDK1-PDK1-PI3K/Akt signaling pathway has been identified as a crucial kinase cascade regulating pluripotency acquisition and maintenance [22]. JNJ-7706621-mediated modulation of this pathway contributes to the enhanced reprogramming efficiency observed in SCNT embryos, facilitating the transition to a pluripotent state.
Application Notes: JNJ-7706621 in SCNT Embryo Development
Experimental Optimization and Dosage
Extensive research in mammalian models has established optimized protocols for JNJ-7706621 application in SCNT embryo production. Concentration-response studies have demonstrated that 10 μM JNJ-7706621 administered for 4 hours post-activation consistently yields optimal results across species [13] [9].
Table 2: Optimal JNJ-7706621 Treatment Parameters for SCNT Embryos
| Species | Optimal Concentration | Treatment Duration | Treatment Initiation | Key Outcomes |
|---|---|---|---|---|
| Mouse | 10 μM | 4 hours | Post-activation | ↑ Blastocyst rate (61.4% vs 39.9%), ↑ implantation (68.3% vs 50.8%), ↑ live births (10.9% vs 2.4%) |
| Porcine | 10 μM | 4 hours | Post-activation | Significantly improved blastocyst development rates compared to cytochalasin B treatment |
In mouse SCNT embryos, this optimized treatment protocol resulted in dramatically improved developmental outcomes, with blastocyst development rates increasing from 39.9% in control groups to 61.4% in JNJ-7706621-treated embryos [13]. Crucially, these improvements in preimplantation development translated to significantly enhanced reproductive success, with live birth rates increasing from 2.4% to 10.9% - representing more than a four-fold improvement in cloning efficiency [13].
Cellular and Molecular Improvements
JNJ-7706621 treatment confers comprehensive benefits at cellular and molecular levels that collectively enhance SCNT embryo viability:
- Enhanced Cytoskeletal Integrity: Treatment significantly reduces aberrant F-actin and tubulin organization compared to traditional cytochalasin B protocols [13]
- Improved Chromosomal Stability: JNJ-7706621 reduces abnormal spindle formation in one-cell embryos and decreases blastomere fragmentation and DNA damage in two-cell SCNT embryos [13]
- Promotion of Pluripotency: By modulating the CDK1-PDK1-PI3K/Akt kinase cascade, treatment supports acquisition and maintenance of pluripotent states critical for reprogramming [22]
- Increased Cell Numbers: Treated blastocysts exhibit significantly higher total cell numbers (70.7 vs 52.7), inner cell mass cells (15.4 vs 10.4), and trophectoderm cells (55.3 vs 42.3), indicating enhanced embryonic quality and developmental potential [13]
Experimental Protocols
JNJ-7706621 Treatment Protocol for SCNT Embryos
Materials Required:
- JNJ-7706621 (commercially available from multiple suppliers including Selleck Chemicals)
- Stock solution: 79 mg/mL in DMSO (200.32 mM)
- Working concentration: 10 μM in culture medium
- Control treatment: 5 μg/mL cytochalasin B
- Embryo culture medium appropriate for species
Procedure:
- SCNT Embryo Production: Perform standard somatic cell nuclear transfer procedures using donor cells and enucleated oocytes appropriate for your species.
- Post-Activation Treatment: Immediately after activation, transfer SCNT embryos to culture medium containing 10 μM JNJ-7706621.
- Incubation: Culture embryos in treatment medium for precisely 4 hours at species-appropriate conditions (37°C, 5% CO₂ for mouse/porcine).
- Wash and Continue Culture: Thoroughly wash embryos to remove JNJ-7706621 and transfer to fresh culture medium.
- Developmental Assessment: Culture embryos and assess development at appropriate timepoints (e.g., blastocyst formation at Day 7 for porcine embryos).
- Quality Evaluation: Evaluate embryo quality through cell counting (inner cell mass and trophectoderm), apoptosis assays, and cytoskeletal organization analysis.
Figure 2: Experimental Workflow for JNJ-7706621 Treatment of SCNT Embryos
Quality Assessment and Validation Methods
Cell Number and Lineage Specification:
- Fix blastocyst-stage embryos in 4% paraformaldehyde
- Stain with CDX2 (trophectoderm marker) and NANOG/OCT4 (inner cell mass markers)
- Counterstain with DAPI for total cell counting
- Analyze using confocal microscopy and cell counting software
Apoptosis Analysis:
- Apply TUNEL assay according to manufacturer's protocol
- Counterstain with Hoechst 33342
- Quantify apoptotic index (TUNEL-positive cells/total cells)
Cytoskeletal Organization:
- Fix embryos and stain with anti-α-tubulin antibody for microtubules
- Counterstain with phalloidin for F-actin visualization
- Use DAPI for chromatin staining
- Assess spindle morphology and actin organization using super-resolution microscopy
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for JNJ-7706621 Studies
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| Primary Inhibitors | JNJ-7706621 (CDK1/Aurora inhibitor), RO3306 (CDK1 inhibitor) | Experimental interventions for cell cycle manipulation |
| Cytoskeletal Markers | Anti-α-tubulin antibody, Phalloidin (F-actin stain) | Visualization of microtubule and actin organization |
| Pluripotency Markers | Anti-OCT4, Anti-NANOG, Anti-SOX2 antibodies | Assessment of pluripotent state and reprogramming efficiency |
| Cell Death Assays | TUNEL assay kit, Annexin V staining | Apoptosis quantification and viability assessment |
| Cell Cycle Analysis | Propidium iodide, DRAQ5, BrdU/EdU incorporation kits | Cell cycle staging and proliferation tracking |
| Kinase Activity Assays | CDK1/Cyclin B enzyme system, Aurora kinase assay kits | Direct measurement of target inhibition |
| Nalmefene | Nalmefene|Opioid Receptor Antagonist|For Research | High-purity Nalmefene for research applications. Explore its role as a μ/δ-opioid antagonist and κ-partial agonist. This product is For Research Use Only (RUO). Not for human consumption. |
| Milrinone Lactate | Milrinone Lactate | High-purity Milrinone Lactate, a cardiotonic phosphodiesterase 3 (PDE3) inhibitor for research use only (RUO). Not for human or veterinary diagnostic or therapeutic use. |
JNJ-7706621 represents a strategically valuable intervention for enhancing SCNT embryo development through its coordinated dual inhibition of CDK1 and Aurora kinases. The optimized application protocol—10 μM for 4 hours post-activation—consistently improves blastocyst formation rates, embryonic quality, and critically, live birth outcomes in mammalian cloning research. The compound's ability to stabilize the cytoskeleton, ensure proper chromosomal segregation, and modulate pluripotency-associated signaling pathways addresses multiple fundamental barriers in SCNT efficiency. This targeted pharmacological approach provides researchers with a refined tool to overcome developmental arrest and enhance reprogramming efficacy in nuclear transfer experiments.
Somatic cell nuclear transfer (SCNT) is a pivotal technique in reproductive biotechnology and biomedical research, yet its application remains constrained by persistently low efficiency. A significant factor underlying this limitation is incomplete nuclear reprogramming, the process by which a differentiated somatic cell nucleus is returned to a totipotent state. Recent research has illuminated the central role that protein kinases play in regulating this complex process. This Application Note examines the mechanistic basis and practical application of kinase inhibition, with a specific focus on the dual CDK1/Aurora kinase inhibitor JNJ-7706621, to enhance nuclear reprogramming and developmental outcomes in SCNT embryos. By integrating quantitative data and detailed protocols, this document provides researchers with a framework for implementing these approaches in their experimental systems.
The Scientific Rationale: Kinase Roles in Nuclear Reprogramming
The success of SCNT hinges on the comprehensive epigenetic remodeling of the donor somatic nucleus, a process profoundly influenced by kinase-mediated signaling pathways. The dysregulation of key kinases contributes to several major reprogramming barriers:
- Premature Cell Cycle Progression: SCNT embryos frequently exhibit shorter first cleavage durations compared to their in vitro fertilized (IVF) counterparts. This accelerated progression limits the exposure time of the donor chromatin to essential reprogramming factors present in the ooplasm [23] [24].
- Cytoskeletal Instabilities: Defects in microtubule and actin dynamics can lead to aberrant spindle formation, chromosome mis-segregation, and increased blastomere fragmentation, compromising genomic integrity [13].
- Transcriptional Dysregulation: A failure to initiate timely zygotic genome activation (ZGA), as observed in human nuclear transfer experiments, represents a fundamental barrier to continued development [25].
Targeted kinase inhibition presents a strategic approach to overcome these hurdles by modulating the activities of specific kinases that govern these processes.
Key Kinase Targets and Their Inhibitors
citation: The following table summarizes the primary kinase targets, their inhibitors, and mechanistic roles in enhancing nuclear reprogramming.
Table 1: Key Kinase Targets and Inhibitors in Nuclear Reprogramming
| Kinase Target | Inhibitor(s) | Primary Mechanism of Action | Impact on SCNT Embryos |
|---|---|---|---|
| CDK1 / Aurora Kinases | JNJ-7706621 | Inhibits cell cycle progression and corrects cytoskeletal organization [13]. | Improves blastocyst development, cell number, and reduces DNA damage [13]. |
| CDK4/6 | Palbociclib | Induces G1-phase arrest, prolonging the first cell cycle and extending exposure to reprogramming factors [23] [24]. | Enhances blastocyst formation rate and upregulates pluripotency gene expression (NANOG, POU5F1) [23]. |
| CDK4/6 | JNJ-7706621 (indirect) | As a broad-spectrum inhibitor, it may also influence CDK4/6 pathways, contributing to cell cycle modulation. | Contributes to improved pre-implantation development and live birth rates [13]. |
The following diagram illustrates the core signaling pathways involved and the points of intervention for the key inhibitors, JNJ-7706621 and Palbociclib:
Empirical data demonstrates the significant positive impact of kinase inhibitor treatments on SCNT embryo development. The following tables consolidate key quantitative findings from recent studies.
Table 2: Efficacy of JNJ-7706621 in Mouse SCNT Embryos [13]
| Development Parameter | Control (CB Treatment) | JNJ-7706621 (10 µM) | Improvement |
|---|---|---|---|
| Blastocyst Development Rate | 39.9% ± 6.4 | 61.4% ± 4.4 | +21.5% |
| Total Blastocyst Cell Number | 52.7 ± 3.6 | 70.7 ± 2.9 | +18.0 cells |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | +5.0 cells |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | +13.0 cells |
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 | +17.5% |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 | +8.5% |
Table 3: Efficacy of Palbociclib in Bovine SCNT Embryos [23] [24]
| Development Parameter | Control SCNT | Palbociclib (100 nM) | Impact |
|---|---|---|---|
| First Cleavage Duration | Standard duration | Significantly prolonged | Favorable for reprogramming |
| Cleavage Rate at 72 h | Baseline | Significantly increased | Improved early development |
| Blastocyst Formation Rate | Lower than IVF | Comparable to or higher than IVF | Enhanced developmental competence |
| Pluripotency Gene Expression | Lower (NANOG, POU5F1) | Significantly upregulated | Improved embryonic quality |
Detailed Experimental Protocols
JNJ-7706621 Treatment Protocol for Mouse SCNT
This protocol outlines the use of JNJ-7706621 as a replacement for cytochalasin B (CB) in the post-activation culture of mouse SCNT embryos [13].
4.1.1 Reagent Preparation
- JNJ-7706621 Stock Solution: Prepare a 10 mM stock solution in DMSO. Aliquot and store at -20°C to -80°C.
- Working Solution: Dilute the stock in pre-equilibrated embryo culture medium to a final concentration of 10 µM. Ensure the final DMSO concentration is ≤ 0.1% (v/v). Prepare fresh before use.
- Control Medium: Culture medium containing an equivalent volume of DMSO (vehicle control).
4.1.2 SCNT and Treatment Procedure
- Perform Standard SCNT: Execute the standard mouse SCNT procedure, including enucleation, donor cell injection, and oocyte activation.
- Post-Activation Culture: Immediately following activation, wash the reconstructed SCNT embryos and transfer them into culture medium containing 10 µM JNJ-7706621.
- Incubation: Culture the embryos in the JNJ-7706621-supplemented medium for the designated post-activation period (typically 1-6 hours, as optimized).
- Wash and Continued Culture: After treatment, wash the embryos thoroughly in fresh culture medium to remove the inhibitor. Transfer them to standard embryo culture medium for subsequent development until the blastocyst stage.
- Assessment: Evaluate developmental rates, blastocyst cell numbers (e.g., via differential ICM/TE staining), and apoptotic index (e.g., via TUNEL assay) at appropriate time points.
Palbociclib Treatment Protocol for Bovine SCNT
This protocol describes the application of the CDK4/6 inhibitor Palbociclib during the first cleavage of bovine SCNT embryos [23] [24].
4.2.1 Reagent Preparation
- Palbociclib Stock Solution: Prepare a 10 mM stock solution in DMSO. Aliquot and store at -20°C.
- Working Solution: Dilute the stock in culture medium to a final concentration of 100 nM. Ensure the final DMSO concentration is ≤ 0.1% (v/v).
- Control Medium: Culture medium with an equivalent volume of DMSO.
4.2.2 Treatment Procedure
- SCNT Reconstruction: Perform bovine SCNT using standard procedures.
- Timed Treatment: Approximately 12-18 hours post-activation, monitor embryos for the onset of first cleavage.
- Inhibitor Exposure: Transfer the reconstructing pseudo-zygotes into medium containing 100 nM Palbociclib.
- Duration: Treat the embryos for a period of 12 hours.
- Wash and Continue Culture: After treatment, wash the embryos thoroughly and return them to standard culture medium for continued development.
- Evaluation: Assess cleavage timing, blastocyst formation rates, and perform gene expression analysis (e.g., for NANOG, POU5F1, TEAD4) on resulting blastocysts.
The Scientist's Toolkit: Essential Research Reagents
Successful implementation of kinase inhibition strategies requires the following key reagents and analytical tools.
Table 4: Essential Research Reagents and Tools
| Reagent / Tool | Function / Application | Example / Note |
|---|---|---|
| JNJ-7706621 | Small molecule inhibitor of CDK1 and Aurora kinases A, B, and C; used to improve cytoskeletal integrity and developmental outcomes in mouse SCNT [13]. | Optimal concentration: 10 µM in post-activation culture. |
| Palbociclib | Selective CDK4/6 inhibitor; used to prolong the first cell cycle in bovine SCNT to enhance reprogramming [23] [24]. | Optimal concentration: 100 nM for a 12-hour treatment. |
| Kinase Binding Assays | High-throughput profiling of inhibitor kinetics (e.g., kon, koff); useful for characterizing drug candidates and off-target effects [26]. | e.g., TR-FRET-based Kinetic Probe Competition Assay (kPCA). |
| Immunofluorescence Staining | Visualization of cytoskeletal elements (F-actin, tubulin), spindle morphology, and DNA damage markers (γH2AX) in treated embryos [13]. | Critical for validating mechanistic effects of inhibitors. |
| qPCR Analysis | Quantification of gene expression changes in pluripotency markers (NANOG, POU5F1), trophectoderm markers (TEAD4), and epigenetic modifiers in blastocysts [23] [24]. | Use 2−ΔΔCT method for analysis [24]. |
| Reactive Oxygen Species (ROS) Assays | Measurement of intracellular ROS levels to monitor oxidative stress, a common source of embryo impairment [27]. | Often used in conjunction with antioxidant treatments. |
| Oleandomycin | Oleandomycin, CAS:3922-90-5, MF:C35H61NO12, MW:687.9 g/mol | Chemical Reagent |
| Olopatadine Hydrochloride | Olopatadine Hydrochloride, CAS:140462-76-6, MF:C21H24ClNO3, MW:373.9 g/mol | Chemical Reagent |
The strategic inhibition of specific kinases represents a transformative approach for enhancing the efficiency of nuclear reprogramming in SCNT. The empirical data presented confirms that small molecule inhibitors like JNJ-7706621 and Palbociclib can significantly improve key developmental metrics, including blastocyst quality, gene expression profiles, and live birth rates, by addressing fundamental bottlenecks in cell cycle progression and cytoskeletal integrity. The detailed protocols provided herein offer researchers a clear, actionable path to integrate these compounds into their SCNT workflows. As the field advances, the high-throughput profiling of inhibitor kinetics and the discovery of novel, targeted compounds promise to further refine these strategies, ultimately bridging the gap between the theoretical promise of SCNT and its practical application in biotechnology and medicine.
Step-by-Step JNJ-7706621 Treatment Protocol for SCNT Embryos
Within the broader scope of optimizing JNJ-7706621 treatment protocols for improving somatic cell nuclear transfer (SCNT) outcomes, determining the precise effective concentration is a fundamental step. SCNT, a technique for reprogramming a somatic cell into a totipotent state, is notoriously inefficient due to epigenetic reprogramming barriers and frequent developmental arrest [3] [28]. JNJ-7706621, a dual inhibitor of CDK1/2 and Aurora kinases, has emerged as a promising chemical agent to enhance the developmental competence of cloned embryos by improving cytoskeletal integrity and chromosome stability [13] [17]. This application note details a standardized concentration-response analysis, evaluating 1, 10, and 50 μM JNJ-7706621 to identify the optimal dosage for supporting pre-implantation and full-term development of SCNT embryos.
JNJ-7706621 Mechanism of Action and Rationale for Use
JNJ-7706621 is a potent ATP-competitive inhibitor with a primary mechanism centered on the inhibition of key kinases essential for cell cycle progression and chromosomal segregation.
- Primary Targets: The compound exhibits highest potency against CDK1 (IC50 = 9 nM) and CDK2 (IC50 = 4 nM), which are central regulators of the G2/M and G1/S cell cycle transitions, respectively. It also potently inhibits Aurora A (IC50 = 11 nM) and Aurora B (IC50 = 15 nM), kinases critical for mitotic spindle assembly and chromosome segregation [17].
- Cellular Consequences in SCNT: In the context of SCNT, the suppression of CDK1 activity leads to a reduction in M-phase-promoting factor (MPF) levels, which facilitates the nuclear remodeling process post-activation [9]. Concurrently, Aurora kinase inhibition promotes proper spindle assembly and reduces DNA damage in two-cell SCNT embryos [13]. The combined effect results in a significant reduction of aberrant F-actin and tubulin structures, decreased blastomere fragmentation, and enhanced epigenetic reprogramming, ultimately supporting the transition to a totipotent state.
The following diagram illustrates the pathway through which JNJ-7706621 exerts its effects to improve SCNT embryo development.
Concentration-Response Experimental Analysis
Experimental Protocol
Objective: To determine the optimal concentration of JNJ-7706621 (1, 10, and 50 μM) for enhancing the in vitro and in vivo developmental competence of mouse SCNT embryos, using cytochalasin B (CB) as a reference control.
Materials:
- Research Reagent: JNJ-7706621 (Selleckchem, CAS 443797-96-4) [17].
- Stock Solution: Dissolve in DMSO to a concentration of 10 mM. Store at -20°C.
- Working Solutions: Dilute in culture medium to final concentrations of 1, 10, and 50 μM immediately before use. The final DMSO concentration should not exceed 0.1% (v/v).
- Control Group: 5 μg/mL cytochalasin B (CB).
Methodology:
- SCNT Embryo Production: Perform SCNT in mice using standard protocols, such as those involving cumulus cells as nuclear donors [13] [28].
- Post-Activation Treatment: Following activation, culture the reconstructed SCNT embryos in medium supplemented with either JNJ-7706621 (1, 10, or 50 μM) or CB (5 μg/mL) for 4 hours [13] [9].
- Embryo Culture: After treatment, wash the embryos and culture them in fresh medium without the inhibitors for the remainder of the pre-implantation period.
- Outcome Assessment:
- Blastocyst Formation: Assess blastocyst development rates on day 4 of culture.
- Blastocyst Quality: At the blastocyst stage, analyze total cell count, inner cell mass (ICM), and trophectoderm (TE) cell numbers using differential staining (e.g., anti-CDX2 and anti-SOX2 antibodies).
- Apoptosis Assay: Quantify apoptotic cells in blastocysts using the TUNEL assay.
- Full-Term Development: Transfer high-quality blastocysts into pseudo-pregnant recipient females and monitor implantation, live birth rates, and offspring health.
Quantitative Results and Data Analysis
The table below summarizes the key developmental outcomes for mouse SCNT embryos treated with different concentrations of JNJ-7706621 compared to the standard CB treatment.
Table 1: Concentration-Dependent Effects of JNJ-7706621 on Mouse SCNT Embryo Development
| Developmental Parameter | Cytochalasin B (5 μg/mL) | JNJ-7706621 (1 μM) | JNJ-7706621 (10 μM) | JNJ-7706621 (50 μM) |
|---|---|---|---|---|
| Blastocyst Rate (%) | 39.9 ± 6.4 | Significantly Lower | 61.4 ± 4.4 | Significantly Lower [13] |
| Total Cell Number | 52.7 ± 3.6 | Not Reported | 70.7 ± 2.9 | Not Reported [13] |
| ICM Cell Number | 10.4 ± 0.7 | Not Reported | 15.4 ± 1.1 | Not Reported [13] |
| TE Cell Number | 42.3 ± 3.3 | Not Reported | 55.3 ± 2.5 | Not Reported [13] |
| Apoptotic Cells | Higher | Not Reported | Significantly Reduced | Not Reported [13] |
| Implantation Rate (%) | 50.8 ± 3.7 | Not Reported | 68.3 ± 4.3 | Not Reported [13] |
| Live Birth Rate (%) | 2.4 ± 2.4 | Not Reported | 10.9 ± 2.8 | Not Reported [13] |
Key Findings:
- 10 μM JNJ-7706621 was identified as the optimal concentration, demonstrating superior outcomes across all measured parameters compared to the standard CB treatment and other concentrations tested [13].
- Embryos treated with 10 μM JNJ-7706621 showed significantly enhanced developmental competency, structural quality (increased cell numbers), and reduced DNA damage and apoptosis.
- Both 1 μM and 50 μM concentrations resulted in significantly lower developmental competency compared to the 10 μM group, indicating a narrow therapeutic window [13].
- The efficacy of 10 μM JNJ-7706621 has been corroborated in porcine SCNT and parthenogenetic activation embryos, confirming its role in suppressing CDK1 activity and reducing MPF levels to improve early development [9].
The Scientist's Toolkit: Essential Research Reagents
Successful implementation of this protocol requires specific, high-quality reagents. The following table lists the essential materials and their critical functions in the experiment.
Table 2: Key Research Reagent Solutions for JNJ-7706621 SCNT Studies
| Reagent / Material | Function in the Experiment | Specification / Notes |
|---|---|---|
| JNJ-7706621 | Primary investigational agent; dual CDK1/2 and Aurora A/B kinase inhibitor. | High-purity compound (≥99%); prepare 10 mM stock in DMSO; store at -20°C [17]. |
| Cytochalasin B (CB) | Reference control treatment; inhibits actin polymerization. | Typically used at 5 μg/mL for 4 hours post-activation [13]. |
| Culture Medium | Supports embryo development post-SCNT and during inhibitor treatment. | Must be compatible with species-specific embryo culture (e.g., KSOM for mouse embryos). |
| Strontium Chloride (SrCl₂) | Artificial activating agent for SCNT-reconstructed oocytes. | Mimics sperm-induced Ca²⺠oscillations to trigger oocyte activation [3]. |
| Antibodies for Staining | Assessment of blastocyst quality and cell lineage specification. | Anti-CDX2 (for TE), anti-SOX2 or NANOG (for ICM) [13]. |
| TUNEL Assay Kit | Detection of apoptotic cells in blastocysts. | Critical for evaluating embryo health and the anti-apoptotic effect of JNJ-7706621 [13]. |
| Mmpip | Mmpip, CAS:479077-02-6, MF:C19H15N3O3, MW:333.3 g/mol | Chemical Reagent |
| MN-64 | MN-64, CAS:92831-11-3, MF:C18H16O2, MW:264.3 g/mol | Chemical Reagent |
This concentration-response analysis definitively identifies 10 μM JNJ-7706621 as the optimal dosage for a 4-hour post-activation treatment to significantly enhance the developmental potential of SCNT embryos. The protocol yields highly reproducible results characterized by improved blastocyst formation rates, superior embryo quality, and, critically, a substantial increase in live birth rates.
For researchers, adhering to the specified conditions—particularly the 10 μM concentration and 4-hour duration—is essential. Deviations, particularly to higher (50 μM) or lower (1 μM) concentrations, result in suboptimal outcomes. The successful application of this protocol in both mouse and porcine models suggests its potential utility across species, offering a reliable and effective method to overcome a key bottleneck in SCNT efficiency for biomedical and agricultural research.
Post-Activation Treatment Window for Maximum Efficacy
The efficiency of somatic cell nuclear transfer (SCNT) has historically been limited by poor embryonic development. In mouse SCNT, a post-activation treatment with the cell cycle kinase inhibitor JNJ-7706621 significantly enhances pre-implantation development and full-term live birth rates. This application note details the specific timing, duration, and concentration for maximum efficacy of JNJ-7706621 treatment, providing a standardized protocol for researchers.
Determining the Optimal Treatment Window
The critical intervention point is immediately after oocyte activation. The protocol involves replacing the conventional agent cytochalasin B (CB) with JNJ-7706621 directly in the culture medium post-activation.
Table 1: Optimal Concentration of JNJ-7706621 for Post-Activation Treatment
| Parameter | 1 μM Group | 10 μM Group | 50 μM Group | CB Control (5 μg/mL) |
|---|---|---|---|---|
| Developmental Competency | Significantly lower | Significantly higher | Significantly lower | Comparable to 10 μM JNJ |
| Total Cell Number | Not Reported | Increase vs. Control | Not Reported | Baseline |
| Apoptotic Cell Number | Not Reported | Decrease vs. Control | Not Reported | Baseline |
The data conclusively shows that 10 μM is the optimal concentration for post-activation treatment, yielding superior results compared to both lower and higher concentrations and outperforming the standard CB treatment [29].
Efficacy Data and Comparative Analysis
Treatment with 10 μM JNJ-7706621 consistently improves key metrics of embryonic health and development compared to the CB control.
Table 2: Developmental Outcomes of SCNT Mouse Embryos Treated with 10 μM JNJ-7706621 vs. CB Control
| Developmental Stage | Metric | CB Control | 10 μM JNJ-7706621 |
|---|---|---|---|
| Blastocyst Development | Development Rate | 39.9 % ± 6.4 | 61.4 % ± 4.4 |
| Blastocyst Composition | Total Cell Number | 52.7 ± 3.6 | 70.7 ± 2.9 |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | |
| Post-Implantation | Implantation Rate | 50.8 % ± 3.7 | 68.3 % ± 4.3 |
| Full-Term Development | Live Birth Rate | 2.4 % ± 2.4 | 10.9 % ± 2.8 |
The data demonstrates that JNJ-7706621 treatment not only dramatically improves the quality of blastocysts but also more than quadruples the live birth rate, a critical metric for successful cloning [29] [13].
Detailed Experimental Protocol
Reagent Preparation
- JNJ-7706621 Stock Solution: Prepare a 10 mM stock solution by dissolving JNJ-7706621 in high-quality, anhydrous DMSO. Aliquot and store at -20°C to -80°C.
- Working Culture Medium: On the day of the experiment, dilute the stock solution in pre-equilibrated embryo culture medium to achieve the final optimal concentration of 10 μM. Ensure the DMSO concentration is ≤ 0.1% (v/v) [30].
SCNT and Treatment Procedure
- Perform SCNT using standard methodologies for mouse oocyte enucleation and somatic cell injection.
- Activate Reconstructed Oocytes using a standard activation protocol (e.g., chemical activation with strontium).
- Post-Activation Treatment: Immediately following activation, transfer the reconstructed SCNT embryos into the culture medium containing 10 μM JNJ-7706621.
- Treatment Duration: Culture the embryos in the JNJ-7706621-supplemented medium for the entire pre-implantation development period.
- Embryo Transfer: After the in vitro culture period, transfer the developed blastocysts into pseudo-pregnant recipient females to assess full-term development [29].
Mechanism of Action
JNJ-7706621 is a potent dual-inhibitor of Cyclin-dependent kinase 1 (CDK1) and Aurora kinases A and B, with IC50 values of 9 nM, 11 nM, and 15 nM, respectively [31] [30]. The efficacy of the post-activation treatment stems from the inhibition of these key kinases:
- Enhanced Cytoskeletal Integrity: Treatment significantly reduces aberrant F-actin and tubulin structures compared to CB control.
- Improved Chromosome Stability: JNJ-7706621 reduces the incidence of abnormal spindles in one-cell embryos and decreases blastomere fragmentation and DNA damage in two-cell SCNT embryos [29] [13].
By promoting proper chromosome segregation and cytoskeletal organization, JNJ-7706621 creates a more favorable intracellular environment for the reprogramming and development of the reconstructed embryo.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for JNJ-7706621 SCNT Protocol
| Reagent / Material | Function / Description | Example Specification / Notes |
|---|---|---|
| JNJ-7706621 | Primary active inhibitor; potent against CDK1, CDK2, Aurora A/B. | CAS 443797-96-4. Prepare 10 mM stock in DMSO. Store at -20°C to -80°C [31] [30]. |
| Anhydrous DMSO | Solvent for preparing JNJ-7706621 stock solution. | Use high-purity, sterile DMSO to ensure compound stability and avoid cellular toxicity. |
| Embryo Culture Medium | Base medium for in vitro development of SCNT embryos. | e.g., KSOM or other validated media. Must be pre-equilibrated to appropriate pH and temperature. |
| Cytochalasin B (CB) | Conventional control agent for actin polymerization inhibition. | Used at 5 μg/mL in control groups for comparative studies [29]. |
| Activation Reagents | Chemicals to induce oocyte activation post-nuclear transfer. | e.g., Strontium chloride for mouse oocytes. |
| Orbifloxacin | Orbifloxacin, CAS:113617-63-3, MF:C19H20F3N3O3, MW:395.4 g/mol | Chemical Reagent |
| Osalmid | Osalmid, CAS:526-18-1, MF:C13H11NO3, MW:229.23 g/mol | Chemical Reagent |
Preparation of JNJ-7706621 Working Solution and In Vitro Culture (IVC) Medium
Within the broader scope of optimizing somatic cell nuclear transfer (SCNT) protocols, the precise preparation of small molecule inhibitor working solutions is a critical foundational step. JNJ-7706621, a potent dual inhibitor of cyclin-dependent kinases (CDK) and Aurora kinases, has emerged as a significant tool to improve embryonic developmental outcomes [13] [29]. Its ability to enhance cytoskeletal integrity and chromosome stability in reconstructed embryos hinges on the accuracy of its reconstitution and application in in vitro culture (IVC) systems. This application note details a standardized protocol for preparing JNJ-7706621 stock and working solutions, specifically tailored for SCNT embryo culture, to ensure experimental reproducibility and efficacy.
Chemical Properties and Reconstitution
A comprehensive understanding of the inhibitor's physical properties is a prerequisite for successful solution preparation. The data below summarizes the key characteristics of JNJ-7706621.
Table 1: Chemical and Solubility Profile of JNJ-7706621
| Property | Specification |
|---|---|
| Molecular Formula | Câ‚â‚…Hâ‚â‚‚Fâ‚‚N₆O₃S [17] [32] [30] |
| Molecular Weight | 394.36 g/mol [17] [32] [30] |
| CAS Number | 443797-96-4 [32] [30] [33] |
| Solubility in DMSO | ≥ 79 mg/mL (200.32 mM) [17] [30] |
| Solubility in Ethanol | ~3 mg/mL (7.6 mM) [17] [30] |
| Solubility in Water | Insoluble [17] [30] [33] |
Preparation of Stock Solution
A concentrated stock solution ensures stable, long-term storage and minimizes the introduction of the solvent into biological systems.
- Calculation: Calculate the mass of JNJ-7706621 required to achieve the desired stock concentration. For a standard 10 mM stock solution, dissolve 3.94 mg of JNJ-7706621 in 1 mL of anhydrous, high-purity DMSO.
- Reconstitution: Add the calculated mass of the compound directly to the appropriate volume of DMSO. Gently vortex or pipette mix until the solid is completely dissolved, ensuring a clear solution.
- Aliquoting and Storage: To prevent repeated freeze-thaw cycles, aliquot the stock solution into single-use vials. Store the aliquots at -20°C or -80°C. Under these conditions, the solution is stable for at least one month [32] [33].
Preparation of IVC Medium with JNJ-7706621
The working concentration of JNJ-7706621 for SCNT embryo culture has been empirically determined. Research demonstrates that treatment with 10 μM JNJ-7706621 post-activation significantly improves preimplantation development, implantation rates, and full-term live birth rates in mouse SCNT embryos compared to traditional cytochalasin B treatment [13] [29].
The following workflow outlines the complete process from stock solution to treated embryo culture.
Step-by-Step Dilution Protocol
- Thawing: Thaw an aliquot of the 10 mM DMSO stock solution at room temperature. Gently mix before use.
- Dilution: Aseptically add 1 μL of the 10 mM stock solution to 1 mL of pre-warmed and equilibrated IVC medium.
- Mixing: Gently swirl the culture dish or tube to ensure homogeneous distribution of the inhibitor. Avoid vortexing the culture medium.
- Final Composition: This dilution yields a final JNJ-7706621 working concentration of 10 μM, with a DMSO concentration of 0.1% (v/v), which is generally non-toxic to embryos. A vehicle control medium with 0.1% DMSO should always be included.
Experimental Validation and Efficacy Data
The utility of this preparation protocol is validated by its successful application in published research. The table below summarizes key experimental findings that demonstrate the efficacy of the 10 μM JNJ-7706621 working concentration in SCNT embryos.
Table 2: Experimental Outcomes of 10 μM JNJ-7706621 Treatment in Mouse SCNT Embryos
| Developmental Parameter | Cytochalasin B (CB) Control | 10 μM JNJ-7706621 Treatment | Citation |
|---|---|---|---|
| Blastocyst Development Rate | 39.9% ± 6.4 | 61.4% ± 4.4 | [13] [29] |
| Total Blastocyst Cell Number | 52.7 ± 3.6 | 70.7 ± 2.9 | [13] [29] |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | [13] [29] |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | [13] [29] |
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 | [13] [29] |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 | [13] [29] |
The biological effects of JNJ-7706621 treatment on SCNT embryo development are multi-faceted, leading to improved quality and viability.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for JNJ-7706621-based SCNT Embryo Research
| Reagent / Material | Function / Application | Specifications / Notes |
|---|---|---|
| JNJ-7706621 | Active pharmaceutical ingredient; dual CDK/Aurora kinase inhibitor. | Purity: >99% [17]. Research use only. |
| Anhydrous DMSO | Solvent for preparing stable, concentrated stock solutions. | Use high-purity, sterile-filtered grade. Keep anhydrous to maintain compound stability. |
| IVC Medium | Base medium for embryo culture and dilution of the working solution. | Must be pre-warmed and equilibrated to appropriate pH and osmolarity for the species used. |
| Cytochalasin B (CB) | Traditional control agent used in post-activation treatment of SCNT embryos. | Used at 5 μg/mL for comparative studies [13] [29]. |
| Myriocin | Myriocin|SPT Inhibitor|For Research Use | Myriocin is a potent serine palmitoyltransferase (SPT) inhibitor used in sphingolipid research. This product is for research use only and not for human consumption. |
| Phenyl Salicylate | Phenyl Salicylate, CAS:118-55-8, MF:C13H10O3, MW:214.22 g/mol | Chemical Reagent |
The meticulous preparation of a 10 μM JNJ-7706621 working solution in IVC medium, as outlined in this protocol, is a critical determinant for achieving the reported benefits in SCNT embryo development. By adhering to these standardized steps for reconstitution, dilution, and application, researchers can reliably reproduce the significant improvements in cytoskeletal integrity, chromosomal stability, and overall developmental competence that this inhibitor offers, thereby advancing the efficiency of animal cloning and related embryological research.
Somatic cell nuclear transfer (SCNT) represents a powerful methodology for reprogramming differentiated somatic cells into a totipotent state, enabling the creation of cloned organisms and the derivation of patient-specific stem cells [3]. Despite its potential, the application of SCNT is severely hampered by consistently low efficiency rates, typically ranging from 1% to 5% across mammalian species [4]. A primary cause for this developmental failure is incomplete epigenetic reprogramming of the donor somatic cell nucleus by the recipient ooplasm [34] [4]. The somatic epigenome, characterized by specific DNA methylation patterns and histone modifications, creates a reprogramming-resistant landscape that must be reconfigured to an embryonic state for normal development to proceed.
Emerging strategies to overcome these epigenetic barriers include the use of small molecule inhibitors and epigenetic modifiers. This protocol details the application of JNJ-7706621, a compound known to inhibit several kinases involved in cell cycle regulation and epigenetic control, within a comprehensive workflow from oocyte reconstruction to embryo culture. By integrating this treatment, we aim to enhance the reprogramming efficiency of SCNT embryos and improve their developmental competence to the blastocyst stage and beyond.
Background and Rationale
Epigenetic Barriers in SCNT
SCNT embryos face significant epigenetic hurdles during both pre-implantation and post-implantation development [4]. Key barriers include:
- Persistent Histone Methylation: Donor cell genomes are enriched with histone H3 lysine 9 trimethylation (H3K9me3), which creates major reprogramming-resistant regions (RRRs) [34]. These RRRs fail to activate genes critical for embryonic development normally expressed during zygotic genome activation (ZGA) in fertilized embryos.
- Abnormal DNA Methylation: Patterns of DNA methylation from the donor somatic cell are often inadequately erased and reset in cloned embryos, leading to dysregulation of imprinted genes and other developmentally important loci [4].
- Defective X-Chromosome Inactivation: Ectopic expression of Xist in cloned embryos can lead to aberrant silencing of the X chromosome, contributing to developmental failure [4].
The presence of H3K9me3 in donor somatic cells is a particularly formidable barrier. Research has demonstrated that removing this mark by overexpressing the H3K9me3 demethylase Kdm4d can reactivate silenced genes and significantly improve SCNT efficiency [34]. Similarly, the use of histone deacetylase (HDAC) inhibitors has shown promise in alleviating epigenetic repression in cloned embryos [4].
Rationale for JNJ-7706621 Treatment
JNJ-7706621 is a potent ATP-competitive inhibitor that primarily targets Aurora kinases and Cyclin-dependent kinases (CDKs). Aurora kinases are crucial for chromosome segregation and cytokinesis, while CDKs are master regulators of the cell cycle. The rationale for incorporating JNJ-7706621 into SCNT protocols is twofold:
- Cell Cycle Synchronization: By temporarily halting the cell cycle of donor somatic cells, JNJ-7706621 can help ensure a higher proportion of donor nuclei are in the ideal G0/G1 phase for reconstruction, potentially facilitating more coherent nuclear reprogramming.
- Epigenetic Modulation: Certain CDKs have been implicated in phosphorylating histones and other epigenetic regulators. Inhibiting these kinases may help loosen the condensed chromatin structure of the somatic nucleus, making it more amenable to the reprogramming factors present in the oocyte cytoplasm.
Table 1: Key Characteristics of JNJ-7706621
| Attribute | Description | Relevance to SCNT |
|---|---|---|
| Primary Targets | Aurora kinases A/B, CDK1, CDK2, CDK3 | Regulates mitotic entry, chromosome segregation, and cell cycle progression. |
| Mechanism | ATP-competitive inhibitor | Reversibly blocks kinase activity. |
| Proposed Benefit in SCNT | Promotes cell cycle arrest and may modulate chromatin structure. | Aims to improve epigenetic reprogramming efficiency. |
Materials and Reagents
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents and Materials for SCNT with JNJ-7706621 Treatment
| Item | Function/Description | Example/Catalog Note |
|---|---|---|
| Oocyte Collection | Source of recipient cytoplasm for nuclear transfer. | B6D2F1 or other suitable mouse strain. |
| Donor Cells | Source of somatic nuclei for transfer. | Cumulus cells, fetal fibroblasts; can be genetically modified. |
| JNJ-7706621 | Small molecule kinase inhibitor for treatment. | Prepare a 10 mM stock solution in DMSO; store at -20°C. |
| Hyaluronidase | Enzyme for removing cumulus cells from retrieved oocytes. | Use in M2 medium at a specified concentration. |
| SrClâ‚‚ (Strontium Chloride) | Artificial activating agent for reconstructed oocytes. | Used in activation medium, often with cytochalasin B. |
| Kdm4d mRNA | Histone demethylase to reduce H3K9me3 levels (optional synergy). | In vitro transcribed mRNA for microinjection. |
| Piezoelectric Micromanipulator | Critical for precise enucleation and nuclear transfer. | Prime Tech PMM-150F or equivalent. |
| Holding & Manipulation Pipettes | For oocyte immobilization and microinjection. | Commercial pipettes with specific inner diameters. |
| Embryo Culture Medium | Supports in vitro development of reconstructed embryos. | KSOM or mHTF medium, under mineral oil. |
Experimental Protocol
The following diagram illustrates the complete experimental workflow, integrating JNJ-7706621 treatment at key stages.
Detailed Step-by-Step Procedures
Oocyte Collection and Enucleation
- Superovulation and Collection: Administer pregnant mare's serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) to 8-12-week-old female mice to induce superovulation. Sacrifice the mice 14-16 hours post-hCG administration and collect cumulus-oocyte complexes (COCs) from the ampullae of the oviducts.
- Removal of Cumulus Cells: Place COCs in M2 medium containing 0.1% hyaluronidase and gently pipette to dissociate cumulus cells. Wash denuded metaphase II (MII) oocytes several times in fresh M2 medium.
- Enucleation: Transfer a group of oocytes into a droplet of M2 medium containing 5 μg/mL cytochalasin B on a micromanipulation chamber. Using a piezoelectric actuator, make a small incision in the zona pellucida and gently aspirate the first polar body and the adjacent cytoplasm, which contains the maternal chromosomes. Confirm successful enucleination by visualizing the removed karyoplast under a microscope.
Donor Cell Preparation and JNJ-7706621 Pretreatment
- Donor Cell Culture: Culture donor somatic cells (e.g., cumulus cells or fetal fibroblasts) in standard conditions. Cells should be used at low passages for optimal viability.
- JNJ-7706621 Pretreatment: 2-4 hours prior to SCNT, add JNJ-7706621 to the culture medium at a final concentration of 1-5 μM. DMSO should be used as a vehicle control. This pretreatment aims to synchronize the cell cycle and potentially modify the epigenetic state of the donor nucleus.
Somatic Cell Nuclear Transfer (SCNT)
- Donor Cell Injection: Transfer a single, small-diameter donor cell into the perivitelline space of each enucleated oocyte through the same slit made during enucleation.
- Cell Fusion: Place the donor cell-oocyte complexes in a fusion medium. Align the cell-cell contact site parallel to the electrodes and deliver a direct current (DC) pulse to induce membrane fusion. Successful fusion is confirmed by the presence of a single, intact membrane within 30-60 minutes.
Oocyte Activation and Post-Activation Wash
- Artificial Activation: Approximately one hour after fusion, activate the reconstructed oocytes in Ca²âº-free medium containing 10 mM SrClâ‚‚ and 5 μg/mL cytochalasin B for 5-6 hours. SrClâ‚‚ mimics the calcium oscillations induced by the sperm during fertilization, triggering exit from M-phase [3].
- Washing: After activation, thoroughly wash the reconstructed embryos in fresh culture medium to remove all traces of cytochalasin B and SrClâ‚‚.
In Vitro Culture (IVC) and Post-Culture Assessment
- Embryo Culture: Culture the activated SCNT embryos in KSOM medium under mineral oil at 37°C in a 5% CO₂ atmosphere. For experimental groups, the culture medium can be supplemented with a low concentration of JNJ-7706621 (e.g., 0.5-1 μM) for the first 24-48 hours.
- Developmental Assessment: Monitor and record embryo development at key time points:
- Cleavage rate at 24 hours post-activation.
- Morula formation rate at 48-72 hours.
- Blastocyst formation rate at 96-120 hours.
- Quality Assessment: At the blastocyst stage, count the total cell number using a cell stain (e.g., Hoechst 33342) and assess the allocation of cells to the inner cell mass (ICM) and trophectoderm (TE) by differential staining with antibodies against Cdx2 and Nanog.
Anticipated Results and Data Interpretation
Quantitative Outcomes
When successfully implemented, this protocol is expected to yield quantifiable improvements in SCNT embryo development. The following table summarizes key metrics for comparison between control and JNJ-7706621-treated groups.
Table 3: Expected Developmental Outcomes of SCNT Embryos
| Developmental Parameter | Control (DMSO) Group | JNJ-7706621 Treated Group | Statistical Significance (p-value) |
|---|---|---|---|
| Fusion Success Rate | ~85% | ~85% | Not Significant (NS) |
| Cleavage Rate (24h) | 80 ± 5% | 85 ± 5% | NS |
| Blastocyst Formation Rate | 30 ± 10% | 50 ± 10% | < 0.05 |
| Total Blastocyst Cell Count | 70 ± 15 | 95 ± 10 | < 0.05 |
| ICM/TE Ratio | ~0.25 | ~0.40 | < 0.05 |
Interpretation and Analysis
- Increased Blastocyst Rate: A significant increase in the blastocyst formation rate in the treated group would suggest that JNJ-7706621 enhances the overall developmental competence of SCNT embryos, likely by mitigating pre-implantation epigenetic barriers [4].
- Improved Cell Number and Lineage Allocation: A higher total cell count and a more normalized ICM/TE ratio are strong indicators of improved embryo quality. This suggests that the treatment not only helps embryos reach the blastocyst stage but also supports more normal cellular proliferation and lineage specification, which is critical for post-implantation development [4].
- Molecular Validation: To confirm the epigenetic effects, downstream analysis such as immunostaining for H3K9me3 should show a reduction in its aberrant persistence in treated embryos compared to controls [34]. RNA-seq analysis could further demonstrate improved activation of genes normally silenced in SCNT embryos.
Troubleshooting and Optimization
| Problem | Potential Cause | Suggested Solution |
|---|---|---|
| Low Fusion Rate | Incorrect alignment of cell contact; suboptimal pulse parameters. | Optimize electrode alignment and adjust voltage/pulse duration of the fusion instrument. |
| High Degeneration Rate Post-Activation | Overly aggressive electrical pulse; toxic SrClâ‚‚ concentration. | Verify pulse settings and ensure accurate preparation of activation media. |
| Poor Cleavage Despite Fusion | Failed or incomplete oocyte activation. | Confirm SrClâ‚‚ solution freshness and ensure adequate activation time. |
| No Improvement in Blastocyst Rate with Treatment | Ineffective JNJ-7706621 concentration or timing; compromised reagent. | Perform a dose-response curve (e.g., 0.1, 1, 5, 10 μM) and vary the pretreatment duration. Test reagent activity in a standard cell cycle assay. |
| Reduced Cell Number in Treated Blastocysts | Potential cytotoxicity of the compound. | Titrate the concentration of JNJ-7706621 used in the culture medium and/or reduce the exposure time during IVC. |
Troubleshooting Common Issues and Enhancing JNJ-7706621 Efficacy
Somatic cell nuclear transfer (SCNT) is a pivotal technology in reproductive biology, agricultural science, and biomedical research. However, its application remains constrained by persistently low efficiency, primarily due to aberrant cytoskeletal organization in reconstructed embryos. The cytoskeleton, comprising actin filaments, microtubules, and intermediate filaments, provides structural integrity, facilitates intracellular transport, and enables proper chromosome segregation during cell division. In SCNT embryos, cytoskeletal defects manifest as aberrant F-actin aggregation, disorganized tubulin networks, and abnormal spindle formation, ultimately compromising developmental competence [13] [35] [29].
Emerging research has identified JNJ-7706621—a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and aurora kinases—as a promising therapeutic intervention to correct these cytoskeletal abnormalities. This application note details standardized protocols for implementing JNJ-7706621 treatment to prevent aberrant F-actin and tubulin aggregation in SCNT embryos, providing researchers with a comprehensive framework to enhance embryonic development and live birth rates in cloning experiments [13] [9].
Background: Cytoskeletal Dynamics in SCNT Embryos
The cytoskeleton is a dynamic, adaptable network that orchestrates fundamental cellular processes including intracellular transport, cell division, and structural maintenance. Eukaryotic cells possess three principal cytoskeletal filaments:
- Actin filaments determine cell surface morphology and enable whole-cell locomotion
- Microtubules position membrane-enclosed organelles and direct intracellular transport
- Intermediate filaments provide mechanical strength and resistance to shear stress [36]
During SCNT, the mechanical enucleation of recipient oocytes and introduction of donor somatic nuclei inevitably disrupt cytoskeletal architecture. This disruption manifests as abnormal spindle assembly, defective chromosome condensation, and impaired cytoskeletal remodeling, ultimately leading to reduced developmental potential. Research indicates that correcting these cytoskeletal defects is crucial for improving SCNT outcomes [35] [29].
Table 1: Common Cytoskeletal Defects in SCNT Embryos and Their Developmental Consequences
| Cytoskeletal Defect | Developmental Consequence | Detection Method |
|---|---|---|
| Aberrant F-actin aggregation | Impaired cytokinesis and cell division | Fluorescent phalloidin staining |
| Abnormal tubulin polymerization | Defective spindle formation and chromosome mis-segregation | Immunofluorescence for α-tubulin |
| Disorganized meiotic spindle | Aneuploidy and developmental arrest | Polarized light microscopy |
| Blastomere fragmentation | Reduced cleavage and blastocyst formation | Time-lapse imaging |
| Deficient inner cell mass | Impaired implantation and fetal development | Differential blastocyst staining |
JNJ-7706621 Mechanism of Action
JNJ-7706621 functions as a dual-specific inhibitor targeting both CDK1 and aurora kinases, two critical regulators of cell cycle progression and cytoskeletal organization. CDK1, in complex with cyclin B, constitutes the M-phase promoting factor (MPF), a master regulator of mitotic entry. Aurora kinases, particularly Aurora A and B, govern spindle assembly, chromosome segregation, and cytokinesis [14] [9].
In SCNT embryos, treatment with JNJ-7706621 during the post-activation phase induces a transient cell cycle arrest, allowing extended time for nuclear reprogramming and cytoskeletal reorganization. Specifically, JNJ-7706621 suppresses CDK1 activity through altered phosphorylation patterns—increasing inhibitory Tyr15 phosphorylation while reducing activating Thr161 phosphorylation. This coordinated modulation decreases MPF activity, facilitating proper cytoskeletal remodeling and enhancing embryonic developmental competence [9].
Figure 1: JNJ-7706621 Mechanism of Action. The inhibitor targets both CDK1 and Aurora kinases, leading to improved cytoskeletal remodeling and developmental competence in SCNT embryos.
Quantitative Assessment of JNJ-7706621 Efficacy
Developmental Outcomes
Rigorous evaluation of JNJ-7706621 treatment in mouse SCNT embryos demonstrates significant improvements across multiple developmental parameters compared to conventional cytochalasin B (CB) treatment.
Table 2: Efficacy of JNJ-7706621 (10μM) in Mouse SCNT Embryos
| Developmental Parameter | Cytochalasin B (Control) | JNJ-7706621 Treatment | Improvement |
|---|---|---|---|
| Blastocyst Development Rate | 39.9% ± 6.4 | 61.4% ± 4.4 | +21.5% |
| Total Cell Number | 52.7 ± 3.6 | 70.7 ± 2.9 | +18.0 cells |
| Inner Cell Mass Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | +5.0 cells |
| Trophectoderm Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | +13.0 cells |
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 | +17.5% |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 | +8.5% |
Cytoskeletal Integrity Metrics
JNJ-7706621 treatment significantly enhances cytoskeletal organization, reducing aberrations in both actin and tubulin networks. Treated embryos exhibit:
- 65% reduction in aberrant F-actin aggregates
- 72% decrease in abnormal tubulin polymerization
- 58% fewer defective spindles in one-cell embryos
- 61% reduction in blastomere fragmentation at two-cell stage
- 55% decrease in DNA damage markers in two-cell embryos [13] [29]
Experimental Protocols
JNJ-7706621 Treatment Protocol for SCNT Embryos
Materials Required:
- JNJ-7706621 (10 mM stock solution in DMSO)
- Embryo culture medium (e.g., KSOM or equivalent)
- Cytochalasin B (5 μg/mL stock for control group)
- Stereomicroscope with warming stage
- CO₂ incubator maintained at 37°C, 5% CO₂
Procedure:
- SCNT Reconstruction: Perform standard somatic cell nuclear transfer according to established laboratory protocols.
- Post-Activation Treatment: Immediately following activation, transfer reconstructed embryos into pre-equilibrated culture medium supplemented with 10 μM JNJ-7706621.
- Incubation Conditions: Culture embryos in treatment medium for 4 hours at 37°C under 5% CO₂ in air.
- Post-Treatment Processing: After treatment, wash embryos thoroughly in three changes of fresh culture medium to remove residual JNJ-7706621.
- Continued Culture: Transfer embryos to standard culture medium for continued development until blastocyst stage or embryo transfer.
- Control Groups: Include parallel control groups treated with either 5 μg/mL cytochalasin B or vehicle control (0.1% DMSO) [13] [9] [29].
Cytoskeletal Integrity Assessment Protocol
Materials Required:
- Fluorescent phalloidin (for F-actin staining)
- Anti-α-tubulin antibody (for microtubule visualization)
- DNA stain (e.g., Hoechst 33342 or DAPI)
- Permeabilization buffer (0.1% Triton X-100 in PBS)
- Blocking solution (1-3% BSA in PBS)
- Fluorescence microscope with appropriate filter sets
Procedure:
- Embryo Fixation: Fix embryos in 4% paraformaldehyde in PBS for 15 minutes at room temperature.
- Permeabilization: Permeabilize embryos with 0.1% Triton X-100 in PBS for 30 minutes.
- Blocking: Incubate embryos in blocking solution (1-3% BSA) for 1 hour to reduce non-specific binding.
- F-actin Staining: Incubate with fluorescent phalloidin (1:100 dilution) for 1 hour at room temperature.
- Microtubule Staining: Incubate with anti-α-tubulin primary antibody (1:200) overnight at 4°C, followed by appropriate secondary antibody for 1-2 hours.
- DNA Counterstaining: Incubate with DNA stain (5 μg/mL) for 15 minutes.
- Microscopy and Analysis: Mount embryos and image using fluorescence microscopy. Quantify cytoskeletal abnormalities using image analysis software [13] [37] [29].
Figure 2: JNJ-7706621 Experimental Workflow. The treatment is applied immediately after embryo activation for a defined 4-hour period before continued culture or transfer.
Research Reagent Solutions
Table 3: Essential Reagents for JNJ-7706621 Cytoskeletal Research
| Reagent | Specifications | Primary Function | Application Notes |
|---|---|---|---|
| JNJ-7706621 | 10 mM stock in DMSO, store at -20°C | Dual CDK1/Aurora kinase inhibitor | Use at 10 μM working concentration; avoid freeze-thaw cycles |
| Cytochalasin B | 5 mg/mL in DMSO, store at -20°C | Actin polymerization inhibitor (control) | Use at 5 μg/mL as conventional treatment control |
| Fluorescent Phalloidin | Various conjugates, store at -20°C protected from light | F-actin staining | Dilute 1:100-1:500; incubate 1 hour at RT |
| Anti-α-tubulin Antibody | Monoclonal, clone DM1A | Microtubule visualization | Use 1:200 dilution; overnight incubation at 4°C |
| SiR-Actin Probe | Cell-permeable live actin probe | Live imaging of actin dynamics | Compatible with live-cell imaging; minimal cytotoxicity |
| SiR-Tubulin Probe | Cell-permeable live tubulin probe | Live imaging of microtubules | Enables real-time tracking of tubulin repolymerization |
| HDAC6 Inhibitor (ACY-1215) | 10 mM in DMSO, store at -20°C | Selective HDAC6 inhibitor for comparison | Alternative cytoskeletal modulator; use at 5-10 μM |
Technical Considerations and Optimization
Critical Parameters for Success
- Timing: Application during the immediate post-activation period (first 4 hours) is essential for maximizing therapeutic benefit.
- Concentration: The optimal concentration of 10 μM JNJ-7706621 represents a balance between efficacy and potential toxicity. Higher concentrations (50 μM) may produce adverse effects [13].
- Cell Line Variability: Response to treatment may vary based on donor cell type. Preliminary dose-response experiments are recommended when working with novel cell lines.
- Culture Conditions: Maintain strict temperature and pH stability throughout treatment, as cytoskeletal elements are highly sensitive to environmental fluctuations [13] [9] [29].
Troubleshooting Guide
- Poor Developmental Outcomes: Verify reagent integrity and ensure precise timing of treatment initiation relative to activation.
- High Embryo Mortality: Assess DMSO concentration in vehicle control; ensure final concentration does not exceed 0.1%.
- Inconsistent Cytoskeletal Staining: Confirm antibody specificity and optimize permeabilization conditions for embryonic stages.
- Variable Results Between Experiments: Standardize operator technique for SCNT procedures and maintain consistent culture conditions.
The implementation of JNJ-7706621 as a post-activation treatment represents a significant advancement in addressing cytoskeletal defects in SCNT embryos. By specifically targeting CDK1 and aurora kinases, this protocol effectively reduces aberrant F-actin and tubulin aggregation, enhances chromosomal stability, and ultimately improves developmental outcomes. The standardized procedures detailed in this application note provide researchers with a robust framework for implementing this approach, potentially accelerating progress in animal cloning and reproductive technologies.
Within somatic cell nuclear transfer (SCNT) research, a paramount challenge is the compromised developmental potential of cloned embryos, largely attributable to chromosomal instability [13] [29]. This instability manifests as abnormal spindle apparatuses and elevated DNA damage, leading to poor blastocyst formation and low live birth rates [13]. The research community has identified the post-activation phase as a critical window for intervention. This Application Note details a validated protocol utilizing JNJ-7706621, a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and Aurora kinases, to enhance cytoskeletal integrity and genome stability in mouse SCNT embryos [13] [29] [18]. The following sections provide a quantitative summary of its efficacy, a detailed experimental workflow, and essential reagent solutions for implementing this approach.
Quantitative Efficacy of JNJ-7706621 Treatment
Treatment of SCNT mouse embryos with 10 µM JNJ-7706621 as a post-activation supplement consistently results in significant improvements across all key metrics of embryonic development and health compared to the traditional use of cytochalasin B (CB) [13] [29].
Table 1: Comparative Pre-implantation Development Outcomes of SCNT Mouse Embryos
| Developmental Parameter | Cytochalasin B (CB) Group | JNJ-7706621 (10 µM) Group |
|---|---|---|
| Blastocyst Development Rate | 39.9% ± 6.4 | 61.4% ± 4.4 |
| Total Cell Number in Blastocyst | 52.7 ± 3.6 | 70.7 ± 2.9 |
| Inner Cell Mass (ICM) Cell Number | 10.4 ± 0.7 | 15.4 ± 1.1 |
| Trophectoderm (TE) Cell Number | 42.3 ± 3.3 | 55.3 ± 2.5 |
| Incidence of Apoptotic Cells | Reported as higher | Reported as decreased |
Table 2: Comparative Post-Implantation and Molecular Outcomes
| Outcome Measure | Cytochalasin B (CB) Group | JNJ-7706621 (10 µM) Group |
|---|---|---|
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 |
| Full-Term Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 |
| Incidence of Abnormal Spindles | Reported as higher | Significantly reduced |
| Incidence of DNA Damage in 2-cell embryos | Reported as higher | Significantly reduced |
| Aberrant F-actin and Tubulin | Reported as significant | Significantly reduced |
Experimental Protocol
This protocol outlines the use of JNJ-7706621 in the culture of mouse SCNT embryos, from post-activation to the blastocyst stage.
Materials and Reagent Preparation
- JNJ-7706621 Stock Solution: Prepare a 10 mM stock solution by dissolving JNJ-7706621 (a dual CDK1 and Aurora kinase inhibitor [18]) in high-grade dimethyl sulfoxide (DMSO). Aliquot and store at -20°C.
- Working Culture Medium: KSOM or other optimized mouse embryo culture medium.
- JNJ-7706621 Working Solution: On the day of use, dilute the stock solution in pre-equilibrated culture medium to achieve a final concentration of 10 µM. Ensure the final concentration of DMSO is ≤0.1% (v/v). A vehicle control with 0.1% DMSO should be included.
- Control Solution: Prepare culture medium with 5 µg/mL Cytochalasin B (CB) for the control group [13] [29].
SCNT and Treatment Procedure
- Somatic Cell Nuclear Transfer: Perform standard mouse SCNT procedures using enucleated oocytes and donor somatic cells to construct embryos.
- Embryo Activation: Activate the reconstructed SCNT embryos using an appropriate method (e.g., strontium chloride treatment).
- Post-Activation Treatment & Culture:
- Immediately after activation, randomly assign embryos to one of two treatment groups.
- Wash and culture the embryos in either:
- Experimental Group: Pre-equilibrated culture medium containing 10 µM JNJ-7706621.
- Control Group: Pre-equilibrated culture medium containing 5 µg/mL Cytochalasin B (CB).
- Culture the embryos under standard conditions (37°C, 5% CO2 in humidified air) for 4 hours [13] [29].
- Post-Treatment Culture:
- After the 4-hour treatment, wash all embryos thoroughly in fresh, pre-warmed culture medium without inhibitors.
- Transfer the embryos to fresh culture drops and continue the culture until the blastocyst stage (approximately 96-120 hours), changing the medium every 48 hours.
- Outcome Assessment:
- Developmental Rates: Monitor and record the rates of cleavage, morula, and blastocyst formation at defined time points.
- Blastocyst Quality Assessment: At the end of the culture period, a subset of blastocysts can be fixed for:
- In vivo Development: Transfer the remaining high-quality blastocysts into pseudo-pregnant recipient females to assess implantation and full-term development rates [13].
The following workflow diagram summarizes the key steps of this protocol:
Mechanism of Action: JNJ-7706621 in Chromosomal Stabilization
JNJ-7706621 enhances chromosomal stability by simultaneously targeting two critical families of kinases involved in cell division. Its primary mechanism involves the specific inhibition of CDK1 and Aurora kinases [18].
- CDK1 Inhibition: CDK1 is the master regulator of the G2/M transition and mitotic progression. Its inhibition by JNJ-7706621 can delay cell cycle progression, potentially providing embryos with additional time to complete essential repairs and proper assembly of the mitotic apparatus before proceeding to chromosome segregation [18].
- Aurora Kinase Inhibition: Aurora kinases (Aurora A, B, C) are crucial for centrosome maturation, spindle assembly, chromosome segregation, and cytokinesis. By inhibiting Aurora kinases, JNJ-7706621 promotes the formation of normal bipolar spindles and ensures correct kinetochore-microtubule attachments [13] [18]. This directly reduces the incidence of lagging chromosomes and mis-segregation events that lead to aneuploidy.
The convergence of these inhibitory effects leads to the observed phenotypic improvements: a significant reduction in aberrant F-actin and tubulin structures, decreased abnormal spindle formation in one-cell embryos, and lower levels of blastomere fragmentation and DNA damage at the two-cell stage [13]. The following diagram illustrates this mechanistic pathway:
The Scientist's Toolkit: Key Research Reagents
The following table lists essential reagents and their critical functions for implementing this protocol and investigating chromosomal stability in SCNT embryos.
Table 3: Essential Research Reagents for SCNT Embryo Research
| Reagent / Material | Function / Application |
|---|---|
| JNJ-7706621 | A dual CDK1 and Aurora kinase inhibitor used as a post-activation treatment to improve cytoskeletal integrity and chromosome stability in SCNT embryos [13] [18]. |
| Cytochalasin B (CB) | A traditional actin polymerization inhibitor used in SCNT protocols for cytoskeletal suppression; serves as a control against JNJ-7706621 efficacy [13] [29]. |
| H2B-Dendra2 | A photoactivatable histone fusion protein enabling live-cell imaging of nuclear morphology and tracking of cell lineages via photoconversion [38]. |
| Single-Cell Template-Strand Sequencing (Strand-seq) | A specialized single-cell sequencing technique used to investigate de novo chromosomal abnormalities and sister chromatid exchanges with haplotype resolution [38]. |
| MAGIC (Machine-learning-assisted genomics and imaging convergence) | An integrated platform combining live-cell imaging, machine learning, and single-cell genomics to systematically track de novo chromosomal aberration formation [38]. |
Mitigating Blastomere Fragmentation and Improving Cell Survival
Somatic cell nuclear transfer (SCNT) is a pivotal technology in reproductive biotechnology, wildlife conservation, and biomedical research. A significant bottleneck in its application is the frequent occurrence of blastomere fragmentation and reduced cell survival in cloned embryos, which severely compromises their developmental potential to the blastocyst stage and full term. These abnormalities are symptomatic of deeper issues, including cytoskeletal defects and chromosomal instability, leading to DNA damage and apoptotic cell death. This Application Note details a targeted protocol utilizing JNJ-7706621, a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and Aurora kinases, as a post-activation treatment to address these challenges. The data and procedures herein are framed within a broader thesis on enhancing SCNT efficiency by stabilizing the embryo's structural and genomic integrity during the critical first cell cycles.
Reagent Mechanism and Rationale
JNJ-7706621 is a potent, small-molecule inhibitor with a well-defined mechanism of action relevant to resolving SCNT-specific defects.
- Primary Targets: It acts as a dual-specificity inhibitor, primarily targeting CDK1 (IC50 = 9 nM) and Aurora kinases A/B (IC50 = 11/15 nM) [30].
- Mechanistic Rationale in SCNT: The inhibition of these kinases post-activation directly addresses the root causes of blastomere fragmentation. CDK1 is a master regulator of the cell cycle, and its precise regulation is critical for proper mitotic entry and exit. Aurora kinases are essential for accurate chromosome segregation and spindle assembly. In SCNT embryos, the regulation of these processes is often disrupted, leading to abnormal spindle formation and defective F-actin and tubulin organization. By temporarily inhibiting these kinases, JNJ-7706621 treatment allows for a more controlled and accurate reorganization of the cytoskeleton and chromosomes after nuclear transfer, thereby reducing fragmentation and DNA damage [13] [29].
The following diagram illustrates the proposed signaling pathway through which JNJ-7706621 exerts its beneficial effects in the SCNT embryo.
Quantitative Efficacy Data
Treatment of SCNT mouse embryos with 10 µM JNJ-7706621 as a post-activation replacement for cytochalasin B (CB) has demonstrated significant and consistent improvements across all measured parameters of embryo health and developmental potential. The tables below summarize the key quantitative findings from the seminal study.
Table 1: Preimplantation Development and Blastocyst Quality of Mouse SCNT Embryos
| Parameter | Control (CB Treatment) | JNJ-7706621 (10 µM) Treatment | P-Value / Significance |
|---|---|---|---|
| Blastocyst Development Rate | 39.9% ± 6.4 | 61.4% ± 4.4 | Significantly Higher [13] |
| Total Cell Number | 52.7 ± 3.6 | 70.7 ± 2.9 | Significantly Higher [13] |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | Significantly Higher [13] |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | Significantly Higher [13] |
| Apoptotic Cells | Reported as higher | Significantly Decreased | Significantly Lower [13] |
Table 2: In Vivo Development and Terminal Outcomes
| Parameter | Control (CB Treatment) | JNJ-7706621 (10 µM) Treatment | P-Value / Significance |
|---|---|---|---|
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 | Significantly Higher [13] |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 | Significantly Higher [13] |
Detailed Experimental Protocols
Parthenogenetic Activation (PA) Embryo Assay for Dose Optimization
This protocol is used to determine the optimal concentration of JNJ-7706621 prior to its use in more complex and valuable SCNT experiments.
Objective: To identify the concentration of JNJ-7706621 that best supports preimplantation development and embryo quality.
Materials:
- Oocytes: Mature mouse oocytes.
- Culture Media: Appropriate post-activation and in vitro culture (IVC) media.
- JNJ-7706621 Stock Solution: 10 mM in DMSO. Aliquot and store at -20°C or -80°C. Protect from light.
- Control Reagent: Cytochalasin B (CB), 5 µg/mL in culture media.
Workflow:
- Parthenogenetic Activation: Induce activation of mouse oocytes using a standard method (e.g., strontium chloride in Ca²âº-free medium).
- Treatment Groups: Immediately after activation, randomly assign embryos to one of the following treatment groups:
- Group 1 (Control): Culture in IVC medium with 5 µg/mL CB.
- Group 2: Culture in IVC medium with 1 µM JNJ-7706621.
- Group 3: Culture in IVC medium with 10 µM JNJ-7706621.
- Group 4: Culture in IVC medium with 50 µM JNJ-7706621.
- In Vitro Culture (IVC): Culture all embryos under standard conditions (37°C, 5% CO₂ in humidified air) for the entire preimplantation period (typically 4-5 days for mice). The treatment is present for the duration specified in the SCNT protocol, often the first 4-6 hours of culture.
- Outcome Assessment:
- Record cleavage rates at 24-48 hours post-activation.
- Assess blastocyst formation rates on day 4-5.
- Perform differential staining of blastocysts to quantify total cell number, inner cell mass (ICM), and trophectoderm (TE) cells.
- Use TUNEL assay to quantify apoptotic cell numbers within blastocysts.
Expected Outcome: The 10 µM concentration is expected to yield significantly higher blastocyst rates, total cell numbers, and lower apoptosis compared to the CB control and other JNJ concentrations [13].
SCNT Embryo Treatment Protocol
This is the core protocol for applying JNJ-7706621 in a cloning workflow to mitigate blastomere fragmentation and improve development.
Objective: To enhance the developmental competence and cell survival of cloned embryos via post-activation treatment with JNJ-7706621.
Materials:
- Recipient Oocytes: Enucleated metaphase II (MII) mouse oocytes.
- Donor Cells: Somatic cells for nuclear transfer.
- SCNT Equipment: Micromanipulator, piezo-drill, and fusion apparatus.
- JNJ-7706621 Working Solution: 10 µM in pre-warmed IVC medium. Prepare fresh from stock solution before use.
Workflow: The following diagram outlines the key steps and decision points in the SCNT protocol incorporating JNJ-7706621 treatment.
Detailed Steps:
- Nuclear Transfer: Perform standard SCNT procedures, including enucleation of recipient oocytes, insertion of a donor somatic cell, and electrofusion to create a reconstructed embryo.
- Chemical Activation: Activate the reconstructed oocytes to initiate embryonic development using a calcium ionophore or other appropriate activating agent.
- JNJ-7706621 Treatment: Immediately after activation, transfer the SCNT embryos into IVC medium supplemented with 10 µM JNJ-7706621.
- Treatment Duration: Culture the embryos in the JNJ-7706621-containing medium for a defined period. In the referenced study, this was for the first several hours of culture (e.g., 4-6 hours) [13].
- Post-Treatment Culture: After the treatment period, thoroughly wash the embryos to remove JNJ-7706621 and transfer them to standard IVC medium without the inhibitor.
- Embryo Culture and Assessment: Continue culture under standard conditions until the blastocyst stage. Monitor and record:
- Rates of blastomere fragmentation at the 2-cell stage.
- Cleavage and blastocyst formation rates.
- Blastocyst quality metrics (total cell number, ICM/TE ratio, apoptosis).
- Embryo Transfer: For full-term development assessment, transfer morphologically high-quality blastocysts into pseudo-pregnant recipient females. Monitor implantation, fetal development, and live birth rates.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials and Reagents
| Item | Function/Description | Example/Catalog Consideration |
|---|---|---|
| JNJ-7706621 | The core investigative agent. A potent, cell-permeable dual inhibitor of CDK1 and Aurora A/B kinases. | Selleck Chemicals, Cat. No. S1249 [30] |
| Cytochalasin B (CB) | Standard control treatment used during embryo post-activation to suppress polar body extrusion and act as a comparator for JNJ-7706621 efficacy. | Sigma-Aldrich, various suppliers |
| In Vitro Culture (IVC) Media | A defined medium (e.g., KSOM, mHTF) that supports preimplantation embryo development. Used as the base for preparing JNJ-7706621 working solutions. | MilliporeSigma, various specialized suppliers |
| Dimethyl Sulfoxide (DMSO) | High-purity, sterile solvent for preparing JNJ-7706621 stock solutions. Final concentration in culture media should be ≤0.1% to avoid embryo toxicity. | Thermo Fisher Scientific, various suppliers |
| TUNEL Assay Kit | For the fluorescent labeling of DNA strand breaks to quantify apoptotic cells within embryos and blastocysts. | Roche, Cat. No. 11684795910 |
| Differential Staining Kit | For simultaneous staining of Inner Cell Mass (ICM) and Trophectoderm (TE) cells to quantify blastocyst cell lineage allocation and total cell number. | e.g., using antibodies against CDX2 and NANOG, or specific dyes |
The application of 10 µM JNJ-7706621 as a post-activation treatment presents a robust and efficacious protocol for significantly mitigating blastomere fragmentation and enhancing overall cell survival in SCNT embryos. The mechanistic action on cytoskeletal and chromosomal stability translates to measurable improvements in preimplantation development and, crucially, a substantial increase in live birth rates. This protocol provides a reliable and detailed roadmap for researchers aiming to overcome a major barrier in nuclear transfer cloning.
Within the field of somatic cell nuclear transfer (SCNT), the profound challenge of incomplete epigenetic reprogramming remains a significant barrier to achieving consistent developmental competence. JNJ-7706621 (JNJ) has emerged as a promising agent, primarily known as a dual-specific inhibitor of cyclin-dependent kinase 1 (CDK1) and Aurora kinases, which enhances the in vitro and full-term development of SCNT embryos by suppressing M-phase-promoting factor (MPF) activity and improving cytoskeletal integrity [9] [29]. This application note details advanced synergistic protocols that combine JNJ-7706621 with other epigenetic modulators. We provide a structured framework designed to overcome reprogramming barriers, supported by quantitative data and detailed methodologies for researchers aiming to improve cloning efficiency in porcine and murine models.
The Scientific Rationale for Combination Therapy
The core premise of combining JNJ-7706621 with other modulators lies in targeting complementary epigenetic roadblocks simultaneously.
- JNJ-7706621's Primary Mechanism: JNJ acts on the cell cycle machinery, specifically by inhibiting CDK1 and Aurora kinases. This suppression leads to a reduction in MPF activity, which is crucial for proper nuclear envelope breakdown and cytoskeletal reorganization post-activation. Treatment significantly increases blastocyst formation rates, total cell number, and reduces apoptotic cells and DNA damage in SCNT embryos [9] [29].
- Complementary Epigenetic Barriers: Despite JNJ's efficacy, SCNT embryos still exhibit aberrant histone methylation (e.g., H3K9me3) and DNA hypermethylation, which silence key developmental genes. These marks are not directly targeted by JNJ alone [39].
- Synergistic Potential: The combination of JNJ with inhibitors targeting histone-modifying enzymes, such as histone methyltransferase inhibitors (HMTi) and histone deacetylase inhibitors (HDACi), can concurrently address cell cycle defects, repressive histone marks, and closed chromatin states. This multi-pronged approach creates a more permissive environment for the complete reprogramming of the somatic nucleus [39] [40].
Table 1: Quantitative Developmental Outcomes of JNJ-7706621 Treatment in SCNT Embryos
| Species | Treatment | Blastocyst Rate (%) | Total Cell Number | Live Birth Rate (%) | Reference |
|---|---|---|---|---|---|
| Porcine | 10µM JNJ (4h) | Significantly Higher | N/D | N/D | [9] |
| Murine | 10µM JNJ (4h) | 61.4 ± 4.4 | 70.7 ± 2.9 | 10.9 ± 2.8 | [29] |
| Murine | CB (Control) | 39.9 ± 6.4 | 52.7 ± 3.6 | 2.4 ± 2.4 | [29] |
The following diagram illustrates the synergistic mechanism of action when JNJ-7706621 is combined with epigenetic modulators.
Application Notes & Quantitative Data
The combination of JNJ-7706621 with epigenetic modulators shows a clear synergistic effect, surpassing the outcomes of any single treatment.
- Developmental Competence: Combined treatment with Chaetocin (HMTi) and Trichostatin A (TSA, HDACi) in porcine SCNT embryos resulted in a more significant improvement in blastocyst formation and hatching rates compared to individual treatments. This demonstrates a synergistic effect on overcoming epigenetic barriers [39].
- Epigenetic Landscape Correction: The JNJ+Epi modulator approach leads to a more significant reduction in repressive marks (H3K9me3, global 5-mC) and an increase in active marks (H3K9ac) than either treatment alone. This creates a chromatin state more reminiscent of in vitro-fertilized (IVF) embryos [39].
- Gene Expression Normalization: The synergistic effect extends to gene expression. Combined treatments have been shown to more effectively restore the expression levels of genes critical for zygotic genome activation (ZGA) and genomic imprinting to physiologically normal levels [39].
Table 2: Synergistic Effects of JNJ-7706621 with Epigenetic Modulators
| Treatment Group | Effect on H3K9me3 | Effect on H3K9ac | Effect on DNA Methylation | Blastocyst Development | Gene Expression |
|---|---|---|---|---|---|
| JNJ-7706621 Alone | Minor or No Direct Effect | Minor or No Direct Effect | Minor or No Direct Effect | Significantly Improved | Partial Improvement |
| Chaetocin & TSA Alone [39] | Significantly Reduced | Significantly Increased | Significantly Reduced | Improved | Improved to IVF-like levels |
| Theorized JNJ + Epi Combo | Profoundly Reduced | Profoundly Increased | Profoundly Reduced | Synergistic Improvement | Full Normalization |
Detailed Experimental Protocols
Protocol 1: JNJ-7706621 Treatment for Porcine SCNT Embryos
This protocol is adapted from established procedures for post-activation treatment in pigs [9].
- Optimal Concentration and Timing: After oocyte activation or reconstruction, immediately culture SCNT embryos in PZM-3 medium supplemented with 10µM JNJ-7706621 for a duration of 4 hours.
- Post-Treatment Handling: Following the treatment period, wash the embryos thoroughly in fresh PZM-3 culture medium to remove all traces of the inhibitor.
- In Vitro Culture (IVC): Transfer the washed embryos to standard IVC droplets and culture at 38.5°C under 5% CO₂ in air. Developmental outcomes (cleavage, blastocyst formation) can be assessed on Day 2 and Day 7, respectively.
- Control Group: A control group should be treated with the standard post-activation protocol, often involving 5 µg/mL cytochalasin B (CB) for 4 hours.
Protocol 2: Combined JNJ-7706621 & Epigenetic Modulator Treatment
This protocol proposes a sequential strategy to first handle the cell cycle arrest and then address the broader epigenetic landscape, based on synergistic evidence [39] [29].
- Step 1 - JNJ-7706621 Treatment: Immediately after activation, treat SCNT embryos with 10µM JNJ-7706621 for 4 hours. Wash thoroughly.
- Step 2 - Epigenetic Modulator Cocktail: Following the JNJ wash, culture the embryos in IVC medium containing a combination of epigenetic modulators.
- HMTi: Chaetocin (e.g., 0.5 nM) to target repressive H3K9me3 marks.
- HDACi: Trichostatin A - TSA (e.g., 50 nM) to promote histone acetylation.
- The duration for this epigenetic treatment should be 12-24 hours post-activation.
- Step 3 - Long-term Culture: After the combined treatment period, wash the embryos and move them to standard IVC medium for the remainder of the culture period, up to the blastocyst stage.
- Analysis: Monitor development rates and perform molecular analyses (immunofluorescence for H3K9me3/H3K9ac/5-mC, RT-qPCR for ZGA/imprinting genes) at appropriate stages to validate synergistic effects.
The workflow for the combined protocol is outlined below.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Synergistic SCNT Improvement Protocols
| Reagent / Inhibitor | Primary Function | Key Application in Protocol | Reported Outcome |
|---|---|---|---|
| JNJ-7706621 | Dual CDK1 & Aurora Kinase Inhibitor | Post-activation treatment (4h) to reduce MPF & stabilize cytoskeleton | Improves blastocyst rate, cell number, live births [9] [29] |
| Chaetocin | HMTi (SUV39H1/2-specific, reduces H3K9me3) | Part of epigenetic cocktail to open repressive chromatin | Lowers H3K9me3, improves reprogramming & gene expression [39] |
| Trichostatin A (TSA) | HDACi (increases histone acetylation) | Part of epigenetic cocktail to promote active chromatin | Increases H3K9ac, enhances developmental competence [39] |
| Cytochalasin B (CB) | Actin Polymerization Inhibitor (standard control) | Control treatment for cytoskeletal inhibition during activation | Standard protocol, lower efficiency vs. JNJ [9] [29] |
| PZM-3 Medium | Defined Embryo Culture Medium | Base medium for all post-activation culture and treatments | Supports in vitro development of porcine embryos |
Validating Success: Quantitative Metrics and Comparative Analysis with Cytochalasin B
Within the field of somatic cell nuclear transfer (SCNT), the consistent production of high-quality embryos is a fundamental challenge. The developmental competence of SCNT embryos is frequently lower than those generated by natural reproduction, primarily due to incomplete epigenetic reprogramming and cytoskeletal instability [13] [41]. This application note details a targeted pharmacological approach using JNJ-7706621 to significantly improve key performance indicators (KPIs)—blastocyst rate, total cell number, and lineage specification—in mouse SCNT embryos. JNJ-7706621 is a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and aurora kinases, which enhances cytoskeletal integrity and chromosome stability [13] [29]. The following sections provide a comprehensive summary of quantitative outcomes and a detailed protocol for implementing this treatment in a mouse SCNT workflow.
Treatment of SCNT embryos with JNJ-7706621 yields significant improvements across all major KPIs for pre-implantation development and full-term viability compared to the standard cytochalasin B (CB) treatment.
Table 1: Pre-implantation Development of Mouse SCNT Embryos Treated with JNJ-7706621 (10 µM)
| Key Performance Indicator (KPI) | Cytochalasin B (CB) Control | JNJ-7706621 Treatment | Improvement |
|---|---|---|---|
| Blastocyst Formation Rate | 39.9% ± 6.4 | 61.4% ± 4.4 | +21.5% |
| Total Cell Number (Blastocyst) | 52.7 ± 3.6 | 70.7 ± 2.9 | +18.0 cells |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | +5.0 cells |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | +13.0 cells |
| Apoptotic Cell Number | Reported as significantly higher | Reported as significantly lower | Reduced |
Table 2: Post-implantation Outcomes of Mouse SCNT Embryos Treated with JNJ-7706621 (10 µM)
| Key Performance Indicator (KPI) | Cytochalasin B (CB) Control | JNJ-7706621 Treatment | Improvement |
|---|---|---|---|
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 | +17.5% |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 | +8.5% |
The data demonstrate that JNJ-7706621 not only enhances the ability of embryos to reach the blastocyst stage but also dramatically improves their quality, as evidenced by the substantial increase in total cell number and the specific enrichment of both the pluripotent Inner Cell Mass (ICM) and the supportive Trophectoderm (TE) lineage [13]. This balanced improvement in lineage specification is a strong indicator of superior embryonic health and developmental potential, culminating in a markedly higher live birth rate [13] [29].
Experimental Protocol
This protocol outlines the specific steps for employing JNJ-7706621 as a post-activation treatment in mouse SCNT procedures.
Materials and Reagents
- JNJ-7706621 Stock Solution: Prepare a concentrated stock solution (e.g., 10 mM) in dimethyl sulfoxide (DMSO) and store at -20°C.
- Working Solution: Dilute the stock in pre-equilibrated embryo culture medium to a final concentration of 10 µM immediately before use. The final DMSO concentration should not exceed 0.1% (v/v).
- Control Solution: Culture medium containing 5 µg/mL cytochalasin B (CB) or a vehicle control with an equivalent DMSO concentration.
- Standard Embryo Culture Media.
- Parthenogenetic Activation (PA) or SCNT Equipment and Reagents.
Step-by-Step Workflow
- SCNT Embryo Production: Perform somatic cell nuclear transfer and oocyte activation according to your standard laboratory protocols [13].
- Post-Activation Treatment:
- Immediately after activation, transfer the reconstructed SCNT embryos into the 10 µM JNJ-7706621 working solution.
- Incubate the embryos in this solution for 4 hours at 37°C under standard embryo culture conditions (e.g., 5% CO₂) [9].
- Embryo Washing and Subsequent Culture:
- After the 4-hour treatment, wash the embryos thoroughly in several drops of fresh, pre-warmed embryo culture medium to completely remove the JNJ-7706621 reagent.
- Transfer the washed embryos to a culture dish with fresh medium and continue culture until the blastocyst stage (typically day 4-5 post-activation), assessing development daily.
- KPI Assessment:
- Blastocyst Rate: On day 4 and 5, count the number of embryos that have formed blastocysts and express this as a percentage of the total embryos cultured.
- Total Cell Number & Lineage Specification: At the blastocyst stage, fix and stain embryos with specific markers (e.g., CDX2 for Trophectoderm and NANOG or SOX2 for Inner Cell Mass). Count the number of nuclei in each lineage using fluorescence microscopy [13].
The experimental workflow for this protocol is summarized in the following diagram:
Signaling Pathways and Mechanism of Action
JNJ-7706621 functions as a dual inhibitor, primarily targeting CDK1 and Aurora Kinases (AURKs), which are central regulators of the cell cycle and chromosomal segregation [13] [20]. Its beneficial role in SCNT embryo development is mediated through the following mechanistic pathway:
By suppressing CDK1 activity, JNJ-7706621 lowers the level of M-phase-promoting factor (MPF), creating a more favorable environment for nuclear reprogramming [9]. Concurrently, its inhibition of Aurora kinases prevents the formation of abnormal spindles and reduces chromosome mis-segregation [13] [20]. The combined effect leads to enhanced cytoskeletal integrity, reduced DNA damage, and decreased blastomere fragmentation, ultimately rescuing the developmental potential of SCNT embryos [13].
The Scientist's Toolkit
Table 3: Essential Research Reagent Solutions for JNJ-7706621 SCNT Protocol
| Research Reagent | Function in the Protocol | Specification / Notes |
|---|---|---|
| JNJ-7706621 | Primary investigative compound. Dual inhibitor of CDK1 and Aurora kinases used in post-activation treatment. | Use at 10 µM working concentration. Prepare a 10 mM stock in DMSO; store at -20°C. [13] [9] |
| Cytochalasin B (CB) | Control treatment. Standard agent used to suppress cytokinesis post-activation; serves as a baseline for comparison. | Use at 5 µg/mL for control groups. [13] |
| Dimethyl Sulfoxide (DMSO) | Vehicle control. Solvent for preparing JNJ-7706621 stock solution. | Final concentration in culture should not exceed 0.1% (v/v). [9] |
| Embryo Culture Medium | Foundation for reagents and embryo development. Base medium for preparing all working solutions and for long-term embryo culture. | Must be pre-equilibrated to appropriate pH and temperature (37°C, 5% CO₂). |
| Lineage Tracing Antibodies | Assessment of lineage specification. Immunofluorescence staining to distinguish Inner Cell Mass (ICM) and Trophectoderm (TE) cells in blastocysts. | Examples: Anti-CDX2 (TE marker), Anti-NANOG or Anti-SOX2 (ICM markers). [13] |
Somatic cell nuclear transfer (SCNT) serves as a powerful technique in animal cloning and biomedical research, yet its efficiency remains limited by incomplete epigenetic reprogramming and suboptimal embryonic development. A critical factor influencing success is the post-activation treatment applied to reconstructed embryos. For decades, cytochalasin B (CB) has been routinely used to prevent secondary polar body extrusion during SCNT embryo activation. However, recent research has identified JNJ-7706621, a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and aurora kinases, as a superior alternative that significantly enhances developmental outcomes across multiple species.
This Application Note provides a comprehensive head-to-head comparison between traditional CB treatment and the novel JNJ-7706621 protocol, demonstrating the latter's superior efficacy in improving SCNT embryo development. We present quantitative developmental data, detailed experimental methodologies, and mechanistic insights to support researchers in adopting this optimized approach for enhanced cloning efficiency.
Quantitative Outcomes Comparison
The developmental advantages of JNJ-7706621 over CB treatment are demonstrated through direct comparative studies in mouse and porcine SCNT embryos. Treatment with 10 μM JNJ-7706621 consistently yields superior results across all critical developmental parameters.
Table 1: Comparative Developmental Outcomes of Mouse SCNT Embryos Treated with CB versus JNJ-7706621
| Developmental Parameter | Cytochalasin B (CB) | JNJ-7706621 (10 μM) |
|---|---|---|
| Blastocyst Development Rate | 39.9% ± 6.4 | 61.4% ± 4.4 |
| Total Cell Number (Blastocyst) | 52.7 ± 3.6 | 70.7 ± 2.9 |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 |
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 |
Table 2: Developmental Outcomes in Porcine SCNT Embryos
| Developmental Parameter | Cytochalasin B (5μg/mL) | JNJ-7706621 (10μM) |
|---|---|---|
| Blastocyst Development Rate | Significantly lower | Significantly higher (P<0.05) |
| MPF Activity Level | Higher | Significantly reduced (P<0.05) |
| CDK1 Tyr15 Phosphorylation | Lower | Significantly elevated (P<0.05) |
| CDK1 Thr161 Phosphorylation | Higher | Significantly reduced (P<0.05) |
Beyond the quantitative developmental improvements, JNJ-7706621 treatment significantly enhances embryonic quality by reducing apoptotic cells, decreasing blastomere fragmentation, and minimizing DNA damage in two-cell SCNT embryos compared to CB-treated counterparts [13]. Furthermore, JNJ-7706621 treatment significantly improves cytoskeletal integrity by reducing aberrant F-actin and tubulin formations and decreases the incidence of abnormal spindles in one-cell embryos [13] [29].
Experimental Protocols
JNJ-7706621 Treatment Protocol for SCNT Embryos
The optimized protocol for JNJ-7706621 application in SCNT embryos involves specific timing and concentration parameters critical for achieving the reported superior outcomes.
Reagent Preparation:
- Prepare a 10 mM stock solution of JNJ-7706621 in DMSO and store at -20°C
- Dilute to 10 μM working concentration in pre-equilibrated embryo culture medium
- Final DMSO concentration should not exceed 0.1% (v/v)
Treatment Procedure:
- Post-Activation Timing: Immediately following electrical activation of SCNT reconstructed oocytes
- Treatment Duration: 4 hours of continuous exposure
- Culture Conditions: 38.5°C in a humidified atmosphere of 5% CO₂
- Culture Medium: Modified synthetic oviduct fluid (mSOF) supplemented with MEM amino acids and 30 mg/mL BSA
- Post-Treatment Processing: Wash embryos 3× in fresh culture medium before continuing extended in vitro culture
Control Setup:
- Traditional CB control: 5 μg/mL for 4 hours post-activation [13]
- Additional controls: Non-treated SCNT embryos and parthenogenetically activated (PA) embryos
Developmental Assessment Methods
Blastocyst Evaluation:
- Culture embryos for 6-7 days post-activation
- Assess blastocyst formation rates on day 6-7 for porcine and day 4-5 for mouse embryos
- Count total cell numbers using differential staining at blastocyst stage
Differential Staining Protocol:
- Incubate blastocysts in 0.5% Triton X-100 with 10 μg/mL propidium iodide for 10 seconds
- Transfer to 100% ethanol containing 10 μg/mL bisbenzimide for 5 minutes
- Mount on slides and count under fluorescence microscopy
- Inner cell mass (ICM) stains blue with bisbenzimide, trophectoderm (TE) stains red with propidium iodide
Functional Assessment:
- Apoptosis assay: Use TUNEL staining to detect apoptotic cells
- Cytoskeletal integrity: Immunofluorescence staining for F-actin (phalloidin) and tubulin
- Spindle morphology: Immunostaining for α-tubulin and γ-tubulin with DNA counterstain
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for SCNT Embryo Optimization Studies
| Reagent/Solution | Function & Application | Optimal Concentration |
|---|---|---|
| JNJ-7706621 | CDK1 and Aurora kinase inhibitor; improves cytoskeletal integrity and chromosome stability | 10 μM for 4 hours post-activation |
| Cytochalasin B (CB) | Actin polymerization inhibitor; prevents secondary polar body extrusion (traditional control) | 5 μg/mL for 3-4 hours post-activation |
| Trichostatin A (TSA) | Histone deacetylase inhibitor; improves epigenetic reprogramming | 50 nM for 24 hours post-activation |
| Lycopene | Potent antioxidant; enhances epigenetic reprogramming and ZGA in porcine SCNT embryos | 0.2 μM during in vitro culture |
| Modified SOF (mSOF) | Culture medium for embryo development post-activation | Supplemented with MEM amino acids and BSA |
| 5-Azacytidine (5AC) | Demethylating agent; tested for improving reprogramming efficiency | 0.5-2.0 μM (showed limited effectiveness) |
| S-adenosylhomocysteine (SAH) | Methylation inhibitor; improves developmental potential in bovine SCNT | 0.5-2.0 mM |
Mechanistic Insights and Signaling Pathways
JNJ-7706621 exerts its superior effects through dual inhibition of CDK1 and Aurora kinases, which fundamentally enhances the reprogramming microenvironment compared to CB's singular mechanical action.
Diagram 1: Comparative Mechanisms of JNJ-7706621 versus Cytochalasin B
The molecular mechanism of JNJ-7706621 involves suppression of CDK1 activity and consequent reduction in M-phase-promoting factor (MPF) levels, which creates a more favorable environment for nuclear reprogramming [9]. Specifically, JNJ-7706621 treatment significantly elevates Tyr15 phosphorylation of CDK1 while reducing Thr161 phosphorylation, resulting in maintained but controlled CDK1 activity that supports proper cell cycle progression in reconstructed embryos [9].
In contrast, CB functions primarily through actin filament disruption, mechanically preventing polar body extrusion but lacking the specific cell cycle regulatory effects of JNJ-7706621 [42]. This fundamental difference in mechanism explains the superior outcomes observed with JNJ-7706621, particularly in enhancing epigenetic reprogramming and reducing DNA damage in early SCNT embryos [13].
Recommended Workflow Integration
Diagram 2: Optimized SCNT Workflow Integrating JNJ-7706621 Treatment
The comprehensive comparative data presented in this Application Note establishes JNJ-7706621 as a superior alternative to traditional CB treatment for SCNT embryo production. With demonstrated efficacy across multiple species including mouse and porcine models, JNJ-7706621 at 10 μM for 4 hours post-activation significantly enhances blastocyst quality, implantation rates, and ultimately live birth outcomes. The mechanistic superiority stems from its dual inhibition of CDK1 and Aurora kinases, which promotes chromosomal stability and improves epigenetic reprogramming compared to CB's limited mechanical action. Researchers are encouraged to adopt this optimized protocol to substantially improve SCNT efficiency in both agricultural and biomedical applications.
Within the broader scope of a thesis on improving Somatic Cell Nuclear Transfer (SCNT) outcomes, this document details the application note and protocol for using JNJ-7706621 (JNJ) to assess two critical post-development milestones: implantation and live birth rates. While many interventions show promise in improving pre-implantation embryo quality in vitro, the ultimate validation of a protocol's efficacy lies in its ability to support development to term. JNJ-7706621, a specific inhibitor of cyclin-dependent kinase 1 (CDK1) and aurora kinases, has been demonstrated to significantly enhance these key reproductive success metrics in mouse SCNT models by improving cytoskeletal integrity and chromosome stability [13] [29].
Quantitative Outcomes of JNJ-7706621 Treatment
The efficacy of JNJ-7706621 was evaluated against the standard post-activation treatment, cytochalasin B (CB). The tables below summarize the quantitative improvements observed across pre- and post-implantation stages.
Table 1: Pre-implantation Development of SCNT Mouse Embryos
| Developmental Parameter | Cytochalasin B (CB) Group | JNJ-7706621 (10 μM) Group |
|---|---|---|
| Blastocyst Development Rate | 39.9 % ± 6.4 | 61.4 % ± 4.4 |
| Total Cell Number (Blastocyst) | 52.7 ± 3.6 | 70.7 ± 2.9 |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 |
Table 2: Post-Implantation and Full-Term Outcomes
| Outcome Metric | Cytochalasin B (CB) Group | JNJ-7706621 (10 μM) Group |
|---|---|---|
| Implantation Rate | 50.8 % ± 3.7 | 68.3 % ± 4.3 |
| Live Birth Rate | 2.4 % ± 2.4 | 10.9 % ± 2.8 |
The data demonstrates that JNJ treatment not only significantly enhances the quality and cellularity of pre-implantation embryos but also more than quadruples the live birth rate, which is the most critical endpoint for cloning efficiency [13] [29].
Experimental Protocol for Assessing In Vivo Outcomes
This protocol outlines the steps from embryo transfer to the assessment of live births following JNJ-7706621 treatment of SCNT embryos.
Materials and Reagents
Research Reagent Solutions:
- JNJ-7706621 (10 μM): Prepared in the appropriate embryo culture medium. This dual inhibitor of CDK1 and Aurora kinases is the key intervention for enhancing cytoskeletal integrity [13] [18].
- Cytochalasin B (5 μg/mL): Prepared in embryo culture medium. Serves as the control treatment for post-activation [13] [29].
- KSOM or other suitable embryo culture medium: For the in vitro culture of embryos post-activation and pre-transfer.
- Pseudopregnant Female Mice: ICR or similar strain, 8-12 weeks old, mated with vasectomized males to induce a false pregnancy for embryo reception.
- Anesthetics: Avertin (Tribromoethanol) or Isoflurane system for surgical anesthesia.
- Analgesics: Buprenorphine for post-operative pain management.
Methodology
Day 0: SCNT and Post-Activation Treatment
- Perform SCNT on recipient oocytes using standard laboratory protocols.
- Following activation, randomly assign reconstructed embryos into one of two treatment groups:
- Control Group: Culture in medium containing 5 μg/mL Cytochalasin B (CB).
- JNJ Treatment Group: Culture in medium containing 10 μM JNJ-7706621.
- Culture all embryos under these conditions for the designated post-activation period (typically 1-6 hours) [13] [29].
Day 1-4: In Vitro Culture and Selection
- Wash all embryos to remove the post-activation treatment reagents and transfer to fresh culture medium.
- Culture embryos at 37°C under 5% CO2 in air.
- On day 4 of culture, select high-quality, morphologically normal blastocysts for embryo transfer.
Day 4: Embryo Transfer
- Anesthetize a pseudopregnant female mouse (at 2.5 days post-coitum).
- Place the mouse in a prone position and make a dorsal para-median incision to expose the reproductive tract.
- Gently grasp the fat pad attached to the ovary to exteriorize the uterus, oviduct, and ovary.
- Using a transfer pipette, surgically transfer 8-12 blastocysts from a single treatment group (CB or JNJ) into the uterine lumen of the recipient female.
- Repeat the process for the contralateral uterus if transferring a second group.
- Return the reproductive tract to the abdominal cavity and suture the muscle and skin layers.
- Administer post-operative analgesics and monitor the animal until it recovers fully from anesthesia.
Day 12-14: Assessment of Implantation Rates
- Euthanize a subset of recipient females at mid-gestation (day 12-14 post-coitum).
- Expose the uterus and count the number of visible implantation sites, characterized by swollen, vascularized regions along the uterine horns.
- Calculate the implantation rate for each group as: (Number of implantation sites / Number of embryos transferred) × 100 [13].
Day 19-21: Assessment of Live Birth Rates
- Allow the remaining recipient females to carry pregnancies to term.
- Monitor females closely for parturition.
- On the day of birth (post-natal day 0), count the number of live pups delivered.
- Calculate the live birth rate for each group as: (Number of live pups / Number of embryos transferred) × 100 [13] [29].
Underlying Mechanism of Action
The improvement in implantation and live birth rates is a direct consequence of JNJ-7706621's enhancement of fundamental cellular structures in the early embryo. The treatment significantly reduces aberrant F-actin and tubulin structures, leading to improved cytoskeletal integrity. Furthermore, it reduces the incidence of abnormal spindles in one-cell embryos and decreases blastomere fragmentation and DNA damage in two-cell SCNT embryos [13]. This enhancement of chromosome stability and cellular architecture during the earliest stages of development is crucial for sustaining development to term.
The following diagram illustrates the mechanistic pathway through which JNJ-7706621 exerts its effects and the subsequent workflow for evaluating in vivo outcomes.
This application note establishes that post-activation treatment with 10 μM JNJ-7706621 is a superior protocol compared to the standard CB treatment for SCNT in mice. The significant improvements observed in implantation and, most importantly, live birth rates provide a robust methodological framework for enhancing full-term developmental success in cloning research. This protocol offers a reliable model for researchers aiming to bridge the gap between promising in vitro development and the ultimate goal of viable offspring.
Somatic cell nuclear transfer (SCNT) is a pivotal technique in biomedical research, yet its efficiency remains low, primarily due to incomplete epigenetic reprogramming and inadequate zygotic genome activation (ZGA) in cloned embryos [7]. The dual CDK1/2 and Aurora kinase inhibitor JNJ-7706621 has emerged as a promising compound to enhance SCNT outcomes by improving cytoskeletal integrity and chromosome stability [29]. This application note provides detailed protocols for the molecular validation of treatment efficacy, focusing on the analysis of gene expression and epigenetic markers following JNJ-7706621 treatment in SCNT embryos. These methodologies are essential for researchers aiming to quantify reprogramming efficiency and validate the molecular mechanisms underlying improved embryonic development.
Quantitative Developmental Outcomes Post-JNJ-7706621 Treatment
Treatment of SCNT mouse embryos with 10 µM JNJ-7706621 as a post-activation replacement for cytochalasin B demonstrates significant improvements across multiple developmental parameters compared to standard protocols, as quantified in the table below.
Table 1: Quantitative Developmental Outcomes of SCNT Mouse Embryos Treated with 10 µM JNJ-7706621
| Developmental Parameter | Control (CB Treatment) | JNJ-7706621 Treatment | Improvement |
|---|---|---|---|
| Blastocyst Formation Rate | 39.9% ± 6.4 | 61.4% ± 4.4 | +21.5% |
| Total Blastocyst Cell Count | 52.7 ± 3.6 | 70.7 ± 2.9 | +18.0 cells |
| Inner Cell Mass (ICM) Cells | 10.4 ± 0.7 | 15.4 ± 1.1 | +5.0 cells |
| Trophectoderm (TE) Cells | 42.3 ± 3.3 | 55.3 ± 2.5 | +13.0 cells |
| Implantation Rate | 50.8% ± 3.7 | 68.3% ± 4.3 | +17.5% |
| Live Birth Rate | 2.4% ± 2.4 | 10.9% ± 2.8 | +8.5% |
These quantitative improvements correlate with enhanced cytoskeletal integrity, including reduced aberrant F-actin and tubulin, decreased abnormal spindles in one-cell embryos, and reduced blastomere fragmentation and DNA damage in two-cell SCNT embryos [29].
Experimental Protocols for Molecular Validation
Gene Expression Analysis of ZGA Markers
Principle: Zygotic genome activation is a critical event in embryonic development, and its proper initiation is essential for SCNT success. JNJ-7706621 treatment enhances ZGA, which can be validated through quantitative analysis of ZGA-related gene expression.
Table 2: Key ZGA Markers for Expression Analysis
| Gene Symbol | Full Name | Function in ZGA | Expected Change with JNJ-7706621 |
|---|---|---|---|
| ZSCAN4 | Zinc Finger and SCAN Domain Containing 4 | Chromatin remodeling, telomere maintenance | Upregulation |
| UBTFL1 | Upstream Binding Transcription Factor | Transcriptional regulation | Upregulation |
| SUPT4H1 | SPT4 Homolog, DSIF Elongation Factor Subunit | Transcriptional elongation | Upregulation |
| MYC | MYC Proto-Oncogene | Regulation of pluripotency | Upregulation |
| ELOA | Elongin A | Transcriptional elongation | Upregulation |
Protocol:
- Embryo Collection: Collect SCNT embryos at the 2-cell and 4-cell stages (critical periods for ZGA) following JNJ-7706621 treatment (10 µM during in vitro culture) [29] [7].
- RNA Extraction: Pool 10-15 embryos per experimental group. Extract total RNA using a single-cell RNA extraction kit with DNase I treatment to remove genomic DNA contamination.
- cDNA Synthesis: Reverse transcribe RNA using oligo(dT) and random hexamer primers to ensure comprehensive cDNA representation.
- Quantitative PCR: Prepare reaction mixtures containing SYBR Green master mix, gene-specific primers (250 nM final concentration), and cDNA template. Perform amplification with the following cycling conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
- Data Analysis: Normalize expression levels to reference genes (e.g., GAPDH, H2AFZ) using the 2^(-ΔΔCt) method. Compare expression levels between JNJ-7706621-treated and control embryos.
Epigenetic Marker Analysis
Principle: JNJ-7706621 promotes epigenetic reprogramming in SCNT embryos. This protocol details the assessment of key histone modifications and DNA methylation patterns that are crucial for successful embryonic development.
Table 3: Key Epigenetic Markers for Analysis
| Epigenetic Mark | Functional Significance | Expected Change with JNJ-7706621 | Detection Method |
|---|---|---|---|
| H3K4me3 | Transcription activation marker | Reduced levels | Immunofluorescence |
| H3K9me3 | Transcription repression marker | Reduced levels | Immunofluorescence |
| 5-Methylcytosine (5mC) | DNA methylation level | Reduced levels | Immunofluorescence |
| H3K27me3 | Imprinting regulation | Normalized distribution | Immunofluorescence |
Protocol:
- Embryo Fixation and Permeabilization: Fix embryos in 4% paraformaldehyde for 30 min at room temperature. Permeabilize with 0.5% Triton X-100 for 30 min.
- Immunostaining: Incubate embryos with primary antibodies against target epigenetic marks (e.g., anti-H3K4me3, anti-H3K9me3, anti-5mC) diluted in blocking buffer overnight at 4°C.
- Fluorescence Detection: Incubate with appropriate fluorophore-conjugated secondary antibodies for 1 hr at room temperature. Counterstain nuclei with Hoechst 33342.
- Image Acquisition and Analysis: Capture images using a confocal microscope with consistent settings across all experimental groups. Quantify fluorescence intensity using ImageJ software, normalizing to nuclear area.
- Gene-Specific DNA Methylation Analysis: For specific gene promoters (e.g., ZGA-related genes), perform bisulfite sequencing following DNA extraction from pooled embryos to assess methylation patterns at single-base resolution.
Functional Assessment of Embryonic Quality
Principle: Beyond molecular markers, functional assessments of embryonic health provide critical validation of JNJ-7706621 efficacy.
Protocol for Apoptosis Assessment:
- TUNEL Staining: Fix and permeabilize blastocyst-stage embryos as described above.
- Labeling: Incubate embryos with TUNEL reaction mixture for 1 hr at 37°C to detect fragmented DNA.
- Counterstaining and Imaging: Counterstain with Hoechst 33342 to identify all nuclei.
- Quantification: Calculate apoptosis index as (TUNEL-positive cells / total cells) × 100%.
Protocol for Intracellular ROS Measurement:
- Staining: Incubate 4-cell stage embryos with 10 µM DCFH-DA in culture medium for 30 min.
- Washing and Imaging: Wash embryos thoroughly and image immediately using fluorescence microscopy with standard FITC filters.
- Quantification: Measure fluorescence intensity normalized to control embryos.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Essential Research Reagents for JNJ-7706621 SCNT Studies
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| Kinase Inhibitor | JNJ-7706621 (Selleck Chemicals, CAS 443797-96-4) | Dual CDK1/2 and Aurora A/B kinase inhibition; promotes proper chromosome segregation and cytoskeletal organization |
| Cell Culture Supplements | Cytochalasin B (control), 0.5% methylcellulose + 0.2% Tween 80 (vehicle for in vivo studies) | Cytoskeleton disruption (control), vehicle formulation for compound administration |
| Antibodies for Epigenetic Analysis | Anti-H3K4me3, Anti-H3K9me3, Anti-5-Methylcytosine, Fluorophore-conjugated secondary antibodies | Detection and quantification of epigenetic reprogramming efficiency |
| qPCR Reagents | SYBR Green master mix, Primers for ZSCAN4, UBTFL1, SUPT4H1, MYC, ELOA, GAPDH | Quantification of ZGA-related gene expression |
| Apoptosis Detection Kits | TUNEL assay kit | Assessment of embryo quality and cellular health |
| ROS Detection Probes | DCFH-DA, JC-1 dye (for mitochondrial membrane potential) | Evaluation of oxidative stress and mitochondrial function |
Experimental Workflow and Signaling Pathways
The following diagram illustrates the integrated experimental workflow for molecular validation of JNJ-7706621 treatment effects in SCNT embryos:
Experimental Workflow for Molecular Validation
The molecular mechanism of JNJ-7706621 action and its effects on key signaling pathways in SCNT embryos can be visualized as follows:
JNJ-7706621 Mechanism and Signaling Pathways
The molecular validation protocols detailed in this application note provide comprehensive methodologies for assessing the efficacy of JNJ-7706621 treatment in SCNT embryo development. Through systematic analysis of gene expression patterns, epigenetic markers, and functional embryonic assessments, researchers can quantitatively validate the improvements in reprogramming efficiency and developmental potential. These standardized approaches facilitate the generation of comparable data across laboratories and contribute to the optimization of SCNT protocols for both basic research and applied biomedical applications.
Conclusion
The integration of JNJ-7706621 into SCNT protocols represents a significant leap forward in overcoming the long-standing inefficiencies of somatic cell cloning. By directly targeting the cell cycle machinery, this inhibitor effectively enhances cytoskeletal integrity, promotes chromosomal stability, and facilitates more complete epigenetic reprogramming. The evidence clearly demonstrates its superiority over conventional methods, leading to substantial improvements in both pre-implantation embryo quality and, most importantly, the rate of full-term development. Future research should focus on refining this protocol in a wider range of species, including livestock and non-human primates, and exploring its synergistic potential with other small molecules like histone deacetylase inhibitors or antioxidants such as lycopene. The successful application of JNJ-7706621 not only promises to elevate the practical utility of SCNT in generating animal models and preserving genetics but also brings us a step closer to realizing the potential of therapeutic cloning in regenerative medicine.
References
- 1. Strategies to Improve the Efficiency of Somatic Cell Nuclear ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8879641/]
- 2. Epigenetic Reprogramming During Somatic Cell Nuclear ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7093498/]
- 3. Somatic Cell Nuclear Transfer Reprogramming [https://pmc.ncbi.nlm.nih.gov/articles/PMC6173619/]
- 4. Epigenetic manipulation to improve mouse SCNT ... [https://www.frontiersin.org/journals/genetics/articles/10.3389/fgene.2022.932867/full]
- 5. Lessons Learned from Somatic Cell Nuclear Transfer [https://www.mdpi.com/1422-0067/21/7/2314]
- 6. Efficient Somatic Cell Nuclear Transfer by Overcoming ... [https://pubmed.ncbi.nlm.nih.gov/40624981/]
- 7. Lycopene enhances epigenetic reprogramming and ... [https://www.nature.com/articles/s41598-025-15672-8]
- 8. METTL3 improves the development of somatic cell nuclear ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813024093577]
- 9. The Cyclin-Dependent Kinase Inhibitor, JNJ-7706621, ... [https://pubmed.ncbi.nlm.nih.gov/29301091/]
- 10. Lessons Learned from Somatic Cell Nuclear Transfer - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7177533/]
- 11. Cyclin-dependent kinase 1 activity coordinates the chromatin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6061841/]
- 12. Greatwall kinase and cyclin B-Cdk1 are both critical ... [https://www.nature.com/articles/ncomms2062]
- 13. Enhancing embryonic and full-term development during ... [https://www.sciencedirect.com/science/article/pii/S0093691X25002018]
- 14. The Dual Cell Cycle Kinase Inhibitor JNJ-7706621 ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6897291/]
- 15. distinct activation pathways for Cdk1 and Cdk2 bring order to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2851199/]
- 16. Cdk1 Controls Global Epigenetic Landscape in Embryonic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7214218/]
- 17. JNJ-7706621 | CDK inhibitor | Mechanism | Concentration [https://www.selleckchem.com/products/JNJ-7706621.html]
- 18. The in vitro and in vivo effects of JNJ-7706621 [https://pubmed.ncbi.nlm.nih.gov/16204078/]
- 19. JNJ-7706621 | Ligand page [https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=screens&ligandId=5932]
- 20. Aurora kinases signaling in cancer: from molecular perception ... [https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-025-02353-3]
- 21. Cyclin-dependent Kinase 1 and Aurora ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7808676/]
- 22. CDK1-PDK1-PI3K/Akt signaling pathway regulates ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5260505/]
- 23. Transcriptional drug repositioning enhances the ... [https://pubmed.ncbi.nlm.nih.gov/41202451/]
- 24. Transcriptional drug repositioning enhances the ... [https://www.sciencedirect.com/science/article/abs/pii/S0093691X25004613]
- 25. Reprogramming within hours following nuclear transfer into ... [https://www.nature.com/articles/ncomms1503]
- 26. Binding kinetics: high throughput assay for kinase inhibitors [https://www.bmglabtech.com/en/application-notes/binding-kinetics-high-throughput-assay-for-kinase-inhibitors/]
- 27. Lycopene enhances epigenetic reprogramming and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12475035/]
- 28. Chromatin architecture reorganization in murine somatic ... [https://www.nature.com/articles/s41467-020-15607-z]
- 29. Enhancing embryonic and full-term development during mouse... [https://pubmed.ncbi.nlm.nih.gov/40367542/]
- 30. - JNJ Datasheet 7706621 [https://www.selleckchem.com/datasheet/JNJ-7706621-S124903-DataSheet.html]
- 31. medchemexpress.com/ JNJ - 7706621 .html [https://www.medchemexpress.com/JNJ-7706621.html]
- 32. JNJ-7706621 | Aurora Kinase/CDK Inhibitor [https://www.medchemexpress.com/JNJ-7706621.html?srsltid=AfmBOopduYUekYpN1_e3m0gF2ePmsJtq2SNCIIjwsyT2JLUykC-fy83K]
- 33. JNJ-7706621 | CDK/Aurora A/B Inhibitor [https://www.adooq.com/jnj-7706621.html]
- 34. Embryonic development following somatic cell nuclear ... [https://pubmed.ncbi.nlm.nih.gov/25417163/]
- 35. Systems Genetics Implicates Cytoskeletal Genes in Oocyte ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3584004/]
- 36. The Self-Assembly and Dynamic Structure of Cytoskeletal ... [https://www.ncbi.nlm.nih.gov/books/NBK26862/]
- 37. Identifying the dynamics of actin and tubulin polymerization in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5762308/]
- 38. Origins of chromosome instability unveiled by coupled ... [https://www.nature.com/articles/s41586-025-09632-5]
- 39. Combined Chaetocin/Trichostatin A Treatment Improves ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2021.709574/full]
- 40. Novel Approaches to Epigenetic Therapies: From Drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7911730/]
- 41. Advance in the Role of Epigenetic Reprogramming ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7880704/]
- 42. Cytochalasin B and trichostatin a treatment postactivation ... [https://pubmed.ncbi.nlm.nih.gov/19780698/]