PAR Proteins and Gastrulation: Orchestrating Cell Polarity and Movement in C. elegans Development
This article synthesizes current research on the fundamental role of PAR proteins in controlling gastrulation in C.
PAR Proteins and Gastrulation: Orchestrating Cell Polarity and Movement in C. elegans Development
Abstract
This article synthesizes current research on the fundamental role of PAR proteins in controlling gastrulation in C. elegans. We explore how this conserved polarity module, first identified for regulating asymmetric cell division, is co-opted to drive the cell shape changes and ingression movements of gastrulation. The content details the molecular circuitry of PAR proteins, their regulation by phosphorylation cycles, and their downstream control of the actomyosin cytoskeleton to power apical constriction. Aimed at researchers and drug development professionals, this review covers foundational principles, advanced imaging and modeling methodologies, common experimental challenges, and comparative analyses that validate PAR protein functions across biological contexts, highlighting their broader implications for understanding cell movement in development and disease.
The PAR Polarity Module: From Asymmetric Division to Gastrulation Control
The discovery of the par (partitioning defective) genes in Caenorhabditis elegans represents a foundational milestone in developmental biology, revealing an ancient and conserved machinery for cell polarization. This whitepaper delineates the historical identification of these genes through innovative genetic screens and explores their profound role in cytoplasmic partitioning and embryonic patterning. Within the broader context of PAR protein function in C. elegans gastrulation research, we examine how these proteins orchestrate critical morphogenetic events, such as endoderm precursor cell (EPC) ingression, via the regulation of apical constriction and actomyosin dynamics. The content is structured to provide researchers, scientists, and drug development professionals with a rigorous technical guide, encompassing quantitative data summaries, detailed experimental methodologies, and essential research tools that have propelled this field forward.
The establishment of cellular polarity is a fundamental process governing asymmetric cell division, cell fate specification, and tissue morphogenesis during embryonic development. In the nematode C. elegans, this process is controlled by an evolutionarily conserved set of proteins known as the PAR (partitioning defective) proteins. The historical discovery of the par genes emerged from a quest to understand how maternal-effect genes control the earliest stages of embryogenesis, particularly the asymmetric partitioning of cytoplasmic components that precedes cell fate determination [1]. These proteins form a core signaling pathway that enables cells to establish and maintain polarized domains, a necessity for processes ranging from asymmetric stem cell divisions to gastrulation movements [1] [2].
Within the specific context of C. elegans gastrulation, PAR proteins play a critical role in coordinating cell ingression and tissue reorganization. Gastrulation involves the internalization of cells that will form internal tissues and organs, with the endoderm precursor cells (EPCs) being the first to ingress at the 26-cell stage [3]. The proper execution of this process relies on the PAR-dependent establishment of cellular asymmetry, which regulates downstream effectors such as the actomyosin cytoskeleton to drive apical constriction and cell movement [3]. This whitepaper will explore the historical linkage between the par genes and cytoplasmic partitioning, and how this foundational polarity system is co-opted to control gastrulation, providing a mechanistic understanding of early embryonic patterning.
Historical Discovery of par Genes
The Genetic Screen: A Landmark Approach
The initial discovery of the par genes was rooted in a pioneering genetic screen conducted by Ken Kemphues and Jim Priess in 1983. The screen was designed to identify maternal-effect genes essential for early embryonic development in C. elegans. A key innovation that enabled this large-scale endeavor was the strategic use of mutant strains with an egg-laying defective (Egl) phenotype. In such strains, embryos that are not released hatch inside their mother and consume her, resulting in a "bag of worms" phenotype. Priess reasoned that by mutagenizing Egl strains, any worm harboring a penetrant maternal embryonic lethal mutation would be spared from being devoured—making it easily identifiable as a surviving, crawling adult on the culture plate [1].
This screening methodology was further streamlined by incorporating a high incidence of males (Him) mutation, which facilitated the maintenance of recessive lethal mutations through heterozygous siblings. In the very first screen employing this strategy, Kemphues and technician Nurit Wolf isolated six embryonic lethal mutants. One strain exhibited a particularly striking phenotype: embryos underwent abnormally equal and synchronous cell divisions, suggesting a profound failure in partitioning cytoplasmic components during early cleavages [1]. This gene was designated par-1, and subsequent screens ultimately identified a total of six core par genes (par-1 to par-6), with multiple alleles isolated for each [1].
Initial Phenotypic Analysis: Revealing the Core Function
Mutant analyses of the identified par genes revealed their fundamental role in two interconnected aspects of cell polarization in the one-cell embryo:
- Asymmetric spindle positioning: The par genes were required for the asymmetric positioning of the mitotic spindle, which results in an unequal first cell division.
- Asymmetric cargo localization: They were essential for the asymmetric positioning of specific proteins and RNAs, such as the germline-specific P-granules, which are critical for establishing distinct cell fates in the daughter cells [1].
Because most par genes functioned upstream of both processes, it was concluded that they encode the core machinery responsible for initiating cell polarization in the C. elegans zygote [1]. This foundational polarity system was later found to operate in numerous other cell types, including those involved in gastrulation, epithelia, and cell migration [1].
Molecular Identities and Conservation of PAR Proteins
The molecular cloning of the six par genes between 1994 and 2002 revealed that they encode components of a novel intracellular signaling pathway, illuminating their potential mechanisms of action [1].
Table 1: Molecular Identities of the Core C. elegans PAR Proteins
| Protein | Molecular Identity | Proposed Function in Polarity |
|---|---|---|
| PAR-1 | Serine/threonine kinase | Posterior determinant; phosphorylates downstream substrates |
| PAR-2 | RING finger domain protein | Potential role in ubiquitination pathway; posterior cortex |
| PAR-3 | PDZ domain protein | Scaffold protein; anterior determinant |
| PAR-4 | Serine/threonine kinase | Kinase; symmetrically localized |
| PAR-5 | 14-3-3 protein | Binds phosphorylated serines/threonines |
| PAR-6 | PDZ domain protein | Scaffold protein; anterior determinant |
| aPKC (PKC-3) | Atypical Protein Kinase C | Kinase; anterior determinant |
The identities of these proteins suggested they formed a complex signaling network. PAR-3 and PAR-6, with their PDZ domains, could function as a scaffold. PAR-1 and PAR-4 are kinases, and PAR-5, a 14-3-3 protein, often recognizes phospho-epitopes, indicating a network regulated by phosphorylation [1]. A critical turning point was the discovery that the fly polarity protein Bazooka is a PAR-3 homolog, and that mammalian PAR-3 binds to an atypical PKC (aPKC) [1]. This was swiftly followed by the identification of C. elegans aPKC (PKC-3) as a protein with a Par phenotype and asymmetric localization [1]. Subsequently, PAR-3, PAR-6, and aPKC were found to form a physical complex with the small GTPase CDC-42, an ancient polarity protein [1] [2]. The high conservation of all PAR proteins across animal species underscored their status as fundamental players in cell polarization [1].
PAR Protein Dynamics and Domain Maintenance
A key question in the field has been how the asymmetric distributions of PAR proteins are maintained stably over time. Research has revealed that PAR proteins are not statically anchored but exist in a dynamic steady state.
A Dynamic Steady State Governed by Diffusion and Antagonism
Fluorescence Recovery After Photobleaching (FRAP) experiments during the maintenance phase of polarity demonstrated that both anterior (PAR-6) and posterior (PAR-2) PAR proteins undergo rapid exchange between the cytoplasm and the membrane, and are free to diffuse laterally within the membrane [4]. This creates a continuous flux of molecules across the boundary between the anterior and posterior domains due to diffusion down their concentration gradients. The stable maintenance of these domains, therefore, does not rely on diffusion barriers or active transport but is instead achieved by a balance of diffusive flux and actin-independent differences in the effective membrane affinities of the PAR proteins between the two domains [4]. Essentially, mutual antagonism between the anterior and posterior PAR complexes creates regional differences in net association and dissociation rates, counteracting the homogenizing effect of lateral diffusion.
Network of Mutual Antagonism
The stable polarization of the PAR domains is enforced by a network of mutually antagonistic interactions:
- The anterior PAR complex (PAR-3/PAR-6/PKC-3) promotes the dissociation of posterior PAR proteins (PAR-1, PAR-2) from the membrane [2].
- Conversely, the posterior PAR proteins promote the dissociation of the anterior complex from the membrane [2]. For instance, PAR-1 phosphorylates PAR-3 to exclude it from the posterior cortex, and PAR-2 plays a role in dissociating PAR-6 and CDC-42 [2].
- This mutual inhibition is complemented by within-group positive feedback, such as the cooperative membrane binding of PAR-3 and its association with PAR-6 and CDC-42 [2] [5].
This network of interactions allows the system to function as a bistable switch, reinforcing initial asymmetries to create and maintain two distinct cortical domains.
Diagram 1: PAR Protein Mutual Antagonism Network. The core signaling network showing mutual exclusion between anterior and posterior PAR proteins, and the stabilizing role of CDC-42.
PAR Proteins in Gastrulation Control
Within the context of C. elegans gastrulation, the PAR polarity system is co-opted to control the intricate cell movements required for internalizing the endoderm and mesoderm precursors. The gastrulation of the EPCs serves as a paradigm for understanding this control.
Cell Biology of Endoderm Precursor Cell (EPC) Ingression
The internalization of the two EPCs at the 26-cell stage involves several coordinated cell biological events:
- Apical Constriction: The EPCs undergo a dramatic flattening and constriction of their contact-free apical surfaces, driving the cytoplasm towards the inner side of the cell. This process is powered by the actomyosin cytoskeleton, specifically the accumulation and contraction of non-muscle myosin II (NMY-2) and phosphorylated regulatory myosin light chain (p-rMLC) at the apical cortex [3].
- Spreading of Neighboring Cells: As the EPCs constrict apically, neighboring cells (MS mesodermal precursors and the P4 germline precursor) migrate over them to cover the space they vacate. This redistribution of embryonic mass is crucial for liberating interior space within the small blastocoel cavity, accommodating the ingressing cells [3].
- Cell Cycle Expansion: The EPCs exhibit a characteristically extended cell cycle, dividing only after they are fully internalized. This extended cycle is pre-programmed and is critical for efficient ingression, as it allows sufficient time for the cytoskeletal rearrangements to occur. Mutants like gad-1, which cause premature division of the EPCs, result in gastrulation failure [3].
Linking PAR Polarity to Gastrulation Movements
The PAR proteins influence gastrulation through multiple mechanisms. They are involved in contact-induced cell polarity, which helps specify the fate of cells like the EPCs. Furthermore, the PAR pathway regulates the actomyosin forces required for apical constriction. The PAR-6/PKC-3 complex is instrumental in this process. PAR-6, in a complex with PKC-3 and CDC-42, directly or indirectly regulates the activity and localization of non-muscle myosin II, thereby controlling the apical constriction that drives EPC ingression [3]. This demonstrates a direct functional link between the embryonic polarity apparatus and the execution of morphogenetic movements during gastrulation.
Table 2: Key Cell Biological Events in C. elegans EPC Ingression
| Event | Description | Molecular Mediators | Functional Significance |
|---|---|---|---|
| Apical Constriction | Flattening and constriction of the apical cell surface | NMY-2 (Myosin II), p-rMLC, Actin microfilaments, PAR-6/PKC-3 | Generates force to drive cell internalization |
| Spreading of Neighbors | Migration of MS and P4 cells over ingressing EPCs | Unknown guidance cues from EPCs | Creates interior space for ingressing cells |
| Cell Cycle Expansion | Extended cell cycle duration specific to E lineage | GAD-1 (WD repeat protein) | Provides time for cytoskeletal changes to complete |
Experimental Protocols and Methodologies
Key Historical and Contemporary Techniques
The study of PAR proteins and gastrulation has relied on a suite of genetic, cell biological, and quantitative imaging techniques.
1. Original Genetic Screening Protocol:
- Strain Construction: Generate a strain with an egg-laying defective (egl) mutation and a high incidence of males (him) mutation.
- Mutagenesis: Treat worms with a chemical mutagen (e.g., ethyl methanesulfonate).
- Phenotypic Screening: Scan culture plates for surviving, crawling adults (potential carriers of maternal-effect lethal mutations).
- Clone Isolation and Characterization: Isolate putative mutants and characterize their embryonic phenotypes using differential interference contrast (DIC) microscopy to assess defects in asymmetric division and cytoplasmic partitioning [1].
2. FRAP Protocol for PAR Protein Dynamics:
- Sample Preparation: Create a strain expressing a functional GFP-tagged PAR protein (e.g., GFP::PAR-6 or GFP::PAR-2).
- Image Acquisition: Use a confocal or spinning-disk microscope to image live embryos during the polarity maintenance phase.
- Photobleaching: Apply a high-intensity laser pulse to a defined region of interest (ROI) within either the anterior or posterior domain, bleaching the GFP fluorescence.
- Recovery Monitoring: Acquire time-lapse images at short intervals post-bleach to monitor fluorescence recovery.
- Data Analysis: Quantify fluorescence intensity within the bleached ROI over time. Analyze the spatial characteristics of recovery (e.g., edge-first vs. uniform) to determine the mode of mobility (lateral diffusion vs. cytosolic exchange) [4].
3. Quantitative Perturbation-Phenotype Mapping:
- Dosage Perturbation: Use genetic tools (e.g., RNAi, CRISPR-engineered alleles) to create a series of strains with varying dosages of a specific PAR protein.
- Live Imaging: Record high-resolution movies of embryonic development in these strains, focusing on polarity markers, spindle positioning, and cell division asymmetry.
- Image Quantitation: Employ automated or semi-automated image analysis workflows to extract quantitative metrics such as PAR domain size and intensity, asymmetry index (ASI) for cell division, and cell cycle timing.
- Phenotype Mapping: Correlate the quantitative changes in protein dosage with the resulting phenotypic outcomes for individual embryos to build perturbation-phenotype maps and assess robustness [5].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for PAR and Gastrulation Studies
| Reagent / Tool | Function and Application | Key Examples / Notes |
|---|---|---|
| PAR Mutants | Loss-of-function alleles to define gene function | Six core par genes (par-1 to par-6); maternal-effect lethal [1]. |
| GFP/RFP Fusion Proteins | Live imaging of protein localization and dynamics | Functional fusions of PAR-2, PAR-6, NMY-2::GFP [3] [4]. |
| RNA Interference (RNAi) | Targeted gene knockdown | Feeding or injection RNAi to deplete specific PAR proteins [2] [5]. |
| FRAP Setup | Analyzing protein kinetics and mobility | Confocal microscope with laser bleaching capability [4]. |
| Actin/Myosin Inhibitors | Probing cytoskeletal requirements | Latrunculin A (actin depolymerizer); Blebbistatin (myosin inhibitor) [3]. |
| Mathematical Models | Theoretical framework for polarity | PDE models simulating PAR network interactions and feedback [2]. |
| CBS-3595 | CBS-3595, CAS:908380-97-2, MF:C18H17FN4O2S, MW:372.4 g/mol | Chemical Reagent |
| CCT036477 | CCT036477, CAS:305372-78-5, MF:C21H18ClN3, MW:347.8 g/mol | Chemical Reagent |
The historical discovery of the par genes opened a critical window into the molecular mechanisms of cell polarity. From the initial genetic screens that identified mutants defective in cytoplasmic partitioning, to the modern quantitative analyses of protein dynamics and network robustness, research on PAR proteins has consistently provided fundamental insights. This body of work has firmly established that an evolutionarily conserved machinery, centered on the PAR proteins, is fundamental for breaking cellular symmetry and orchestrating subsequent developmental events. In C. elegans, this machinery is not only essential for the first asymmetric division but is also intricately linked to the control of gastrulation movements, such as EPC ingression, by regulating the actomyosin cytoskeleton. The experimental paradigms and reagents developed in this system continue to serve as a powerful toolkit for dissecting the principles of cell polarization, with broad implications for understanding development and disease across metazoans.
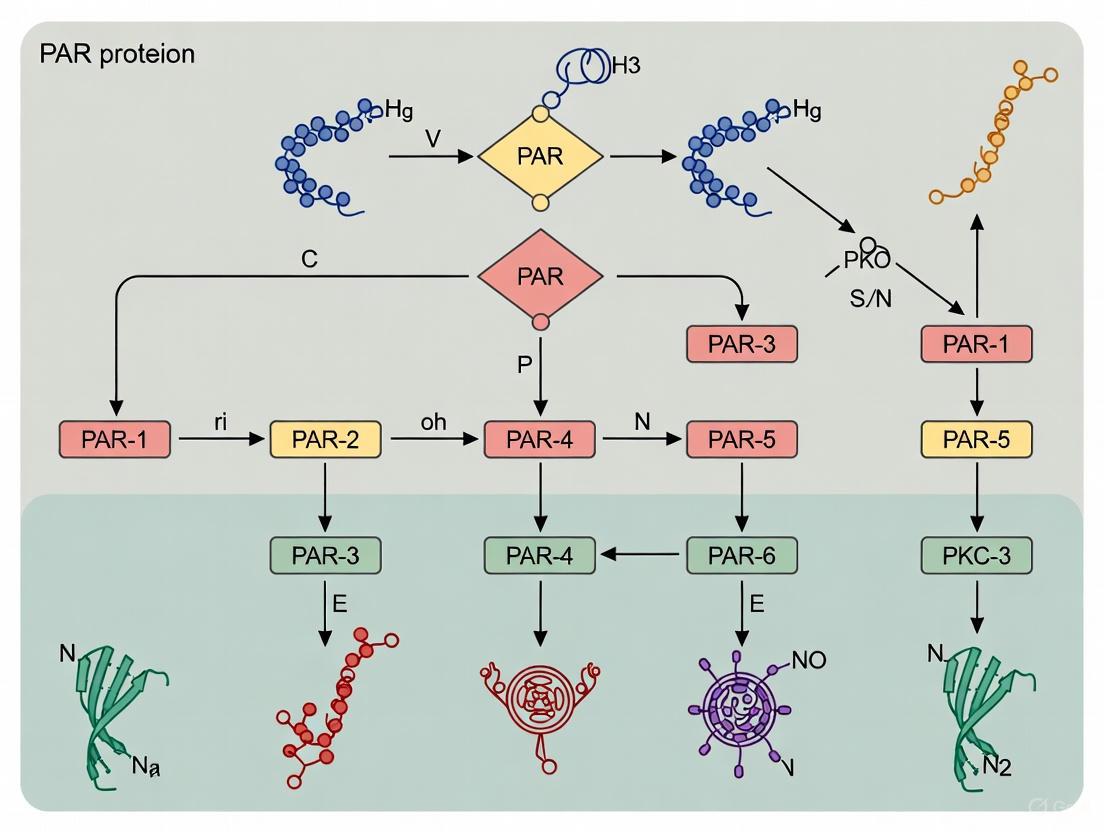
The PAR (Partitioning-defective) proteins constitute an ancient and fundamental mechanism for cell polarization, first discovered in genetic screens for regulators of cytoplasmic partitioning in the early embryo of C. elegans [1]. These proteins are essential for asymmetric cell division, a process critical for generating cell diversity during development. In the C. elegans zygote, PAR proteins become asymmetrically localized to define two opposing cortical domains: an anterior domain (aPAR) containing PAR-3, PAR-6, and PKC-3, and a posterior domain (pPAR) containing PAR-1 and PAR-2 [1] [3]. This polarization establishes a fundamental cellular asymmetry that directs the asymmetric positioning of the mitotic spindle and the unequal segregation of cell fate determinants [1]. The process is not only crucial for the first cell divisions but also for subsequent developmental events, including gastrulation, where contact-induced cell polarity and PAR proteins guide the ingression of endodermal and mesodermal precursor cells [3]. This guide will provide an in-depth technical overview of the core molecular players—the aPAR and pPAR complexes—detailing their conserved domains, functions, and roles in gastrulation, complete with structured data and methodological protocols for researchers.
The Core Molecular Components: aPAR vs. pPAR Complexes
Molecular Identities and Conserved Domains
The six core PAR proteins encoded by the par genes were cloned between 1994 and 2002, revealing that they form a novel intracellular signaling pathway [1]. Their sequences and domain architectures provide critical insight into their functions.
Table 1: Core PAR Proteins and Their Conserved Domains
| Protein | Complex | Conserved Domain(s) | Molecular Function |
|---|---|---|---|
| PAR-3 | aPAR | PDZ domains (multiple) | Acts as a scaffolding protein for complex assembly [1] |
| PAR-6 | aPAR | PDZ domain | Scaffolding protein; binds CDC-42 and PKC-3 [1] |
| PKC-3 | aPAR | Protein Kinase Domain (aPKC) | Serine/Threonine kinase; phosphorylates downstream targets [1] |
| PAR-1 | pPAR | Protein Kinase Domain (Serine/Threonine) | Serine/Threonine kinase; phosphorylates downstream targets [1] |
| PAR-2 | pPAR | RING Finger Domain | Potential E3 ubiquitin ligase activity [1] |
| PAR-5 | Symmetric | 14-3-3 protein domain | Binds phosphorylated serines/threonines; required for mutual exclusion of PAR domains [1] |
| PAR-4 | Symmetric | Protein Kinase Domain (Serine/Threonine) | Serine/Threonine kinase; involved in cell fate specification [1] |
Asymmetric Localization and Mutual Exclusion
The establishment of polarity relies on the mutual exclusion of the aPAR and pPAR complexes from opposing cortical domains. In the one-cell C. elegans embryo, the aPAR complex (PAR-3, PAR-6, PKC-3) becomes enriched in the anterior cortex, while the pPAR complex (PAR-1, PAR-2) localizes to the posterior cortex [1]. PAR-4 and PAR-5 are symmetrically localized, both cortically and cytoplasmically [1]. Genetic analyses have ordered these components into a functional pathway:
- The anterior PAR proteins are required to prevent the posterior PAR proteins from localizing to the anterior.
- Conversely, the posterior PAR proteins prevent the anterior proteins from localizing to the posterior [1].
- PAR-5 is required for the mutual exclusion of these two domains, likely by binding phosphorylated residues generated by the opposing kinases [1].
- In the posterior, the membrane association of PAR-1 is partially dependent on PAR-2 [1].
This antagonistic relationship creates a bistable system that ensures clear demarcation of cellular fronts, a prerequisite for asymmetric division and cell fate specification.
PAR Protein Function in C. elegans Gastrulation
Gastrulation is a critical developmental event during which cells destined to form internal tissues move from the embryo's surface into the interior. In C. elegans, this process begins at the 26-cell stage with the ingression of the two endoderm precursor cells (EPCs) [3]. PAR proteins play a direct role in regulating this cell movement.
The EPCs undergo apical constriction, a process driven by the actomyosin cytoskeleton, which is regulated by PAR proteins. Non-muscle myosin II (NMY-2) and its phosphorylated regulatory light chain (p-rMLC) accumulate at the apical surfaces of the ingressing EPCs [3]. This asymmetric activation of myosin causes cortical microfilaments to contract, flattening and constricting the apical surface and pushing the cytoplasm inward [3]. The regulation of this process is linked to contact-induced cell polarity, which involves PAR proteins. Furthermore, an extended cell cycle in the EPCs, which is crucial for successful ingression, is controlled by genes like gad-1, and this cell cycle expansion is a conserved feature of ingressing cells across species [3].
Experimental Protocols for Key PAR Analyses
Genetic Screens and Mutant Analysis
The original par mutants were identified in maternal embryonic lethal screens in C. elegans [1].
- Methodology: Mutagenized strains with pre-existing egg-laying defective (Egl) and high incidence of male (Him) phenotypes were screened for worms that survived instead of being devoured by their internal progeny. These "escapers" were potential carriers of recessive maternal-effect embryonic lethal mutations [1].
- Phenotypic Analysis: Embryos from identified mutants were examined for defects in early cell divisions. The hallmark "Par" phenotype includes abnormally equal and synchronous early cell divisions, indicating a failure in cytoplasmic partitioning and asymmetric cell division [1].
- Epistasis Analysis: The functional hierarchy of PAR proteins was determined by examining the localization of each PAR protein in the mutant background of others. For example, PAR-1 localization is dependent on PAR-2, and the mutual exclusion of anterior and posterior domains requires PAR-5 [1].
Cell Biological Analysis of Gastrulation
The role of PAR proteins in EPC ingression during gastrulation can be studied using cell biological techniques.
- Cytoskeletal Drug Inhibition: To test the requirement of the actomyosin cytoskeleton in EPC ingression, embryos can be treated with drugs that depolymerize microfilaments (e.g., Latrunculin A) or inhibit myosin activity (e.g., Blebbistatin). These treatments block apical constriction and prevent ingression [3].
- Immunofluorescence and Live Imaging: The apical enrichment of myosin (NMY-2, p-rMLC) in EPCs can be visualized by immunofluorescence. Live imaging of embryos expressing fluorescently tagged PAR proteins, myosin, and actin allows for the real-time observation of polarization and ingression dynamics [3].
- Laser Ablation and Blastomere Manipulation: To understand the role of neighboring cells, the P4 cell (a germline precursor) can be removed via laser ablation. Experiments show that MS cells (mesodermal precursors) can still migrate over the EPCs in the absence of P4, indicating that convergence is guided by cues from the EPCs themselves and not merely by chemotaxis between MS and P4 [3].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagents for PAR Protein and Gastrulation Studies
| Research Reagent | Function/Application in PAR Research |
|---|---|
| PAR Mutants (e.g., par-1, par-2, par-3) | Used for genetic epistasis analysis and to determine the function of each PAR protein in polarization and gastrulation [1]. |
| Anti-PAR Antibodies | Essential for visualizing the asymmetric localization of PAR proteins via immunofluorescence microscopy [1]. |
| Fluorescent Protein Tags (e.g., GFP::PAR-2) | Enable live imaging of PAR protein dynamics and cortical flows in real-time within developing embryos. |
| Cytoskeletal Inhibitors (e.g., Latrunculin A, Blebbistatin) | Used to dissect the functional role of actin and myosin in PAR-dependent processes like apical constriction during gastrulation [3]. |
| Laser Ablation System | Allows for precise killing or cutting of specific cells (e.g., P4) to study their role in cell migration and ingression during gastrulation [3]. |
| K00546 | K00546, CAS:443798-55-8, MF:C15H13F2N7O2S2, MW:425.4 g/mol |
| Cardionogen 1 | Cardionogen 1, CAS:577696-37-8, MF:C13H14N4OS, MW:274.34 g/mol |
Visualizing the PAR Network and Gastrulation
The following diagrams, generated using DOT language, illustrate the core relationships and processes described in this guide.
PAR Protein Polarity Network
PAR Proteins in Gastrulation
The establishment of cellular asymmetry is a fundamental process in developmental biology, directing cell fate specification, tissue morphogenesis, and embryonic patterning. In C. elegans gastrulation, this process is governed by an evolutionarily conserved system of partitioning defective (PAR) proteins, which segregate into antagonistic cortical domains to define the anterior-posterior axis of the embryo [1]. The PAR network, discovered through genetic screens for regulators of cytoplasmic partitioning, comprises six core proteins (PAR-1 to PAR-6) that form a biochemical signaling pathway capable of self-organizing into stable, mutually exclusive membrane domains [1]. This whitepaper examines the principles of mutual antagonism and cortical domain segregation that underpin PAR protein function, focusing on their role in C. elegans gastrulation research and their implications for biomedical applications.
Core Principles of the PAR Polarity System
The Molecular Composition of the PAR Network
The PAR proteins form two functionally and spatially opposed complexes that exhibit mutual antagonism to establish and maintain cellular polarity (Table 1).
Table 1: Core PAR Protein Complexes and Their Functions
| Protein Complex | Component Proteins | Molecular Function | Cortical Localization |
|---|---|---|---|
| anterior PARs (aPARs) | PAR-3, PAR-6, PKC-3 (aPKC) | Scaffold with PDZ domains; serine-threonine kinase; binds CDC-42 | Anterior cortex |
| posterior PARs (pPARs) | PAR-1, PAR-2 | Serine-threonine kinase; RING finger domain | Posterior cortex |
| Ubiquitous PARs | PAR-4, PAR-5 | Serine-threonine kinase; 14-3-3 protein family | Cortical and cytoplasmic |
The anterior PAR complex (PAR-3, PAR-6, and aPKC) localizes to the anterior cortex, while the posterior complex (PAR-1 and PAR-2) occupies the posterior cortex. PAR-4 and PAR-5 remain symmetrically distributed, playing modulatory roles [1]. This asymmetric distribution is established through a combination of mechanical cues and biochemical interactions, culminating in a stable boundary at mid-embryo that persists until cell division.
The Principle of Mutual Antagonism
Mutual antagonism represents the core biochemical principle enabling PAR domain segregation and stability. This reciprocal inhibition occurs through phosphorylation-mediated membrane dissociation:
Anterior-to-posterior inhibition: Membrane-bound aPAR complexes (PAR-3/PAR-6/PKC-3) phosphorylate pPARs (PAR-1 and PAR-2), promoting their dissociation from the membrane and subsequent cytoplasmic localization [2] [6].
Posterior-to-anterior inhibition: Conversely, membrane-associated pPARs phosphorylate aPAR components, triggering their displacement from the cortex [2] [6].
This cross-inhibition creates a self-sustaining system where each complex reinforces its own domain by excluding the opposing complex, forming a sharp boundary at the interface between domains. Genetic evidence demonstrates that disruption of either complex leads to expansion of the other throughout the cortex [4].
Diagram Title: Mutual Antagonism Between PAR Protein Complexes
Dynamic Equilibrium Model of Domain Segregation
The PAR system maintains polarity through a dynamic equilibrium rather than static association. Fluorescence Recovery After Photobleaching (FRAP) experiments reveal that both PAR-2 and PAR-6 undergo continuous exchange between cytoplasmic pools and laterally diffusing membrane-associated states [4]. Several kinetic principles govern this equilibrium:
Free lateral diffusion: PAR proteins freely diffuse within membrane domains, with continuous flux across the boundary due to concentration gradients [4].
Balanced membrane affinity: Spatial differences in effective membrane affinity counterbalance the equalizing effects of lateral diffusion [4].
Actin-independent stabilization: During the maintenance phase, PAR domains remain stable without active actin flows, relying on differences in membrane association/dissociation kinetics [4].
This dynamic system represents a steady state where molecules undergo continuous exchange between regions of net association and dissociation, maintaining stable domains despite constant molecular turnover.
Quantitative Analysis of PAR Protein Dynamics
FRAP Measurements of PAR Protein Kinetics
FRAP experiments provide crucial quantitative insights into PAR protein dynamics during the maintenance phase of polarity (Table 2).
Table 2: Kinetic Parameters of PAR Proteins in C. elegans Embryos
| PAR Protein | Recovery Time (s) | Mobility Mechanism | Dependence on Antagonistic PARs |
|---|---|---|---|
| PAR-6 | ~60 | Lateral diffusion + membrane-cytoplasmic exchange | Requires PAR-2 for anterior restriction |
| PAR-2 | ~60 | Lateral diffusion + membrane-cytoplasmic exchange | Requires PAR-6 for posterior restriction |
| aPKC (PKC-3) | N/A | Complex with PAR-3/PAR-6 | Phosphorylates PAR-1 and PAR-2 |
| PAR-1 | N/A | Membrane association regulated by PAR-2 | Dependent on PAR-2 for cortical localization |
Both PAR-6 and PAR-2 exhibit rapid fluorescence recovery, typically reaching near-complete recovery within 60 seconds post-bleaching. Spatial analysis of recovery patterns demonstrates that both proteins recover more rapidly at the edges of bleached zones than at the center, indicating significant lateral diffusion along the membrane plane [4].
Geometric Control of PAR Domain Positioning
Cell geometry influences PAR patterning through the local membrane-to-cytoplasm ratio, which affects the probability of protein rebinding after dissociation. In prolate spheroid C. elegans embryos (semi-axes: 27 μm major, 15 μm minor), long-axis polarization is favored because it minimizes the interface length between aPAR and pPAR domains, reducing the energetic cost of maintaining the boundary [6]. This geometric sensing is mediated by:
Local surface-to-volume ratio: Higher local membrane curvature affects rebinding probability of dissociated proteins [6].
Interface length minimization: The system naturally evolves toward configurations that minimize the aPAR-pPAR boundary length [6].
Cytosolic dephosphorylation rates: The kinetics of the phosphorylation-dephosphorylation cycle significantly impact axis selection [6].
Experimental Approaches for Investigating PAR Polarity
Genetic Perturbation and Mutant Analysis
Table 3: Key Genetic Approaches in PAR Polarity Research
| Method | Application | Key Findings |
|---|---|---|
| Maternal-effect mutant screens | Identification of core par genes | Discovery of 6 essential par genes [1] |
| RNAi-mediated depletion | Functional analysis of individual PAR proteins | Demonstration of mutual exclusion requirements [4] |
| Transgenic rescue | Structure-function analysis | Determination of functional domains and interactions |
| Conditional mutants (temperature-sensitive) | Analysis of temporal requirements | Separation of establishment vs. maintenance functions [7] |
The initial par mutants were identified through maternal-effect screens in C. elegans, using inventive genetic schemes that took advantage of egg-laying defective (Egl) mutants. In these screens, embryos that failed to hatch inside their mothers would devour them, resulting in "bags of worms" that were easily identifiable [1]. This approach enabled the discovery of the six core par genes, whose mutant phenotypes included abnormally equal and synchronous cell divisions, indicating failed partitioning of cytoplasmic components [1].
Fluorescence Recovery After Photobleaching (FRAP) Protocol
Objective: Quantify mobility and exchange kinetics of PAR proteins during polarity maintenance.
Procedure:
- Sample Preparation: Generate C. elegans strains expressing functional GFP-tagged PAR proteins (GFP::PAR-6 or GFP::PAR-2) that complement native mutations [4].
Imaging Setup:
- Use confocal microscopy with high-sensitivity detectors
- Maintain embryos at 20-25°C during imaging
- Select embryos in maintenance phase (after establishment of PAR domains)
Photobleaching:
- Define region of interest (ROI) within PAR domain
- Apply high-intensity laser pulse (typically 488nm) to bleach fluorescence in ROI
- Ensure bleaching covers entire depth of cortical region
Recovery Imaging:
- Acquire time-lapse images at 1-5 second intervals post-bleach
- Continue imaging for 2-5 minutes to capture complete recovery
- Use low laser power to minimize additional photobleaching
Data Analysis:
- Quantify fluorescence intensity in bleached ROI over time
- Normalize to pre-bleach intensity and correct for background
- Compare recovery kinetics at center vs. edges of bleached zone
- Fit recovery curves to determine diffusion coefficients and exchange rates
Key Controls:
- Perform experiments in wild-type and mutant backgrounds (e.g., par-2(RNAi) for PAR-6 FRAP)
- Verify protein functionality of GFP fusions through complementation tests
- Account for overall photobleaching during time-lapse imaging
Quantitative Imaging and Computational Modeling
Modern analysis of PAR dynamics combines high-throughput imaging with computational modeling. Recent approaches include:
Reaction-diffusion modeling: Partial differential equation models that incorporate realistic cell geometry and biomolecular reactions [2] [6].
Stochastic simulations: Models that account for fluctuations in low-copy number regimes [8].
Sensitivity analysis: Computational approaches to identify critical parameters controlling system behavior [2].
These models have revealed that the phosphorylation-dephosphorylation cycle kinetics and the ratio of membrane-binding to cytosolic diffusion are crucial for robust long-axis polarization [6].
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for PAR Polarity Studies
| Reagent/Tool | Function/Application | Key Features |
|---|---|---|
| GFP::PAR-2/6 fusions | FRAP and live imaging | Functional transgenic fusions for dynamics studies [4] |
| par-2(it5ts) | Temperature-sensitive mutant | Allows temporal control of PAR-2 function [7] |
| RNAi feeding clones | Gene-specific depletion | Enables systematic analysis of protein requirements |
| Anti-PAR antibodies | Immunofluorescence | Fixed analysis of protein localization |
| pMindGFP vector | Conditional antisense expression | Tunable gene suppression [9] |
| Mathematical models | Computational analysis | PDE frameworks for testing hypotheses [2] [6] |
| CE-245677 | CE-245677, CAS:717899-97-3, MF:C24H22Cl2N6O3, MW:513.4 g/mol | Chemical Reagent |
| (R)-CE3F4 | (R)-CE3F4, CAS:143703-25-7, MF:C11H10Br2FNO, MW:351.01 g/mol | Chemical Reagent |
Implications for C. elegans Gastrulation and Beyond
During C. elegans gastrulation, PAR proteins continue to function in polarizing various cell types, including migrating cells and epithelial cells [1]. The balance between different PAR species can specify both asymmetric and symmetric division modes, providing a mechanism for generating cellular diversity during development [10]. Disruption of this balance reprograms division modes independently of cell-size asymmetry or cell-cycle asynchrony, highlighting the fundamental role of PAR-mediated polarity in developmental patterning [10].
The principles of mutual antagonism and cortical domain segregation extend beyond early embryonic patterning to multiple aspects of C. elegans development, including spindle orientation in blastomeres [11] and the regulation of asymmetric cell division by PAR protein modifiers [7]. The conservation of these mechanisms across species underscores their fundamental importance in cell biology and their potential as targets for therapeutic intervention in diseases involving polarized cell processes.
The PAR protein system constitutes an evolutionarily conserved molecular machinery that establishes cellular asymmetry and governs polarized cell behaviors essential for morphogenesis. In C. elegans embryogenesis, PAR proteins not only pattern the anterior-posterior axis in the one-cell embryo but also function reiteratively in subsequent divisions to direct cell fate decisions that ultimately drive gastrulation movements. This whitepaper synthesizes current understanding of how the dynamic interplay between anterior PAR complexes (PAR-3/PAR-6/PKC-3) and posterior PAR proteins (PAR-1/PAR-2) generates molecular asymmetries that specify distinct cell fates, thereby creating the coordinated cellular forces necessary for gastrulation. We present quantitative analyses of PAR protein interactions, detailed experimental frameworks for investigating PAR-mediated morphogenesis, and visualizations of the core regulatory networks that translate cell polarity into tissue remodeling.
Molecular Mechanisms of PAR Protein Function in Cell Polarization
Core PAR Protein Complexes and Their Conserved Roles
The PAR protein network comprises six fundamental components (PAR-1 to PAR-6) that function as master regulators of cell polarity across metazoans. These proteins form two functionally antagonistic groups that establish complementary cortical domains: the anterior PAR complex (PAR-3, PAR-6, and PKC-3) localizes to anterior cortical regions, while the posterior PAR proteins (PAR-1 and PAR-2) occupy posterior domains, with PAR-4 and PAR-5 functioning throughout the cortex and cytoplasm [1]. The molecular identities of these proteins reveal their signaling capabilities: PAR-1 and PAR-4 encode serine-threonine kinases, PAR-5 belongs to the 14-3-3 family of phospho-binding proteins, while PAR-3 and PAR-6 contain PDZ domains that facilitate scaffolding functions [1]. This composition enables the PAR system to integrate spatial information with downstream effector mechanisms.
The evolutionary conservation of PAR proteins underscores their fundamental importance in polarization processes. Following their initial discovery in C. elegans, homologous proteins were identified in Drosophila, where Bazooka (PAR-3 homolog) regulates embryonic polarity, and in mammalian systems, where PAR-3/PAR-6/aPKC complexes control apical-basal polarization in epithelial cells [1]. The core mechanism involves reciprocal inhibition between anterior and posterior PAR complexes, creating a self-reinforcing bistable system that amplifies stochastic fluctuations into stable asymmetries [2].
Downstream Effector Pathways Linking Polarity to Cell Fate
PAR proteins direct cell fate specification through multiple downstream mechanisms that asymmetrically localize cell fate determinants. In the C. elegans embryo, the PAR-1 kinase phosphorylates and regulates the cytoplasmic polyadenylation element binding (CPEB) protein MEX-5, creating a MEX-5/PIE-1 gradient that patterns the anterior-posterior axis [12]. This cytoplasmic asymmetry ensures the differential inheritance of cell fate determinants during successive divisions, ultimately establishing the founder cells that will execute gastrulation movements.
The PAR network also interfaces with cytoskeletal regulators to position mitotic spindles along polarized axes, ensuring asymmetric divisions that generate daughter cells with different sizes and developmental potentials. Recent research has revealed that PAR proteins form apical caps that orient the mitotic spindle in early C. elegans embryos, functioning independently of cell contacts [11]. This spindle orientation mechanism operates in cooperation with the key polarity kinase aPKC (PKC-3 in C. elegans) to coordinate division orientation with the established polarity axis [11].
Quantitative Analysis of PAR Protein Interactions and Dynamics
PAR Protein Localization and Mutual Exclusion Parameters
Table 1: Quantitative Dynamics of PAR Protein Localization in C. elegans Embryos
| PAR Protein | Cortical Localization | Cytoplasmic Pool | Establishment Time | Key Regulators |
|---|---|---|---|---|
| PAR-3 | Anterior cortex | 40-50% | 3-5 minutes | PKC-3, CDC-42 |
| PAR-6 | Anterior cortex | 30-40% | 3-5 minutes | PKC-3, CDC-42, PAR-3 |
| PKC-3 | Anterior cortex | 20-30% | 3-5 minutes | PAR-6, CDC-42 |
| PAR-1 | Posterior cortex | 50-60% | 4-6 minutes | PAR-2, PAR-4 |
| PAR-2 | Posterior cortex | 40-50% | 4-6 minutes | PAR-1, PAR-5 |
| PAR-4 | Uniform cortical | 60-70% | Constitutive | - |
| PAR-5 | Uniform cortical | 70-80% | Constitutive | - |
The dynamic localization patterns of PAR proteins create a molecular asymmetry that guides subsequent developmental processes. During polarization of the one-cell C. elegans embryo, anterior PAR proteins become restricted to the anterior cortex within 3-5 minutes following actomyosin contraction, while posterior PAR proteins occupy the posterior cortical region within 4-6 minutes [13]. The mutual exclusion between these domains is maintained through reciprocal inhibition mechanisms, with PAR-5 (14-3-3 protein) playing a particularly important role in preventing the coexistence of anterior and posterior PAR complexes in the same cortical regions [1].
Biochemical Interaction Network and Feedback Loops
Table 2: Biochemical Interactions in the PAR-CDC-42 Polarity Network
| Interaction | Molecular Mechanism | Functional Outcome | Required Components |
|---|---|---|---|
| aPAR → pPAR inhibition | PKC-3 phosphorylation of PAR-1/PAR-2 | Dissociation of pPAR from membrane | PAR-3, PAR-6, PKC-3 |
| pPAR → aPAR inhibition | PAR-1 phosphorylation of PAR-3 | Dissociation of aPAR from membrane | PAR-1, PAR-2 |
| CDC-42 → aPAR stabilization | GTP-CDC-42 binding to PAR-6 | Enhanced membrane association of aPAR | CDC-42(GTP), PAR-6 |
| aPAR → CDC-42 activation | PAR-6 recruitment of CDC-42 GEF | Local CDC-42 activation | PAR-6, CDC-42 GEF |
| PAR-2 → PAR-1 protection | PAR-2 binding to PAR-1 | Prevents PAR-1 dissociation by aPAR | PAR-2, PAR-1 |
The PAR protein network operates through interconnected feedback loops that create self-sustaining asymmetry. Computational modeling reveals that CDC-42 reinforces maintenance of anterior PAR protein polarity, which in turn feedbacks to maintain CDC-42 polarization, while also supporting posterior PAR protein polarization maintenance [2]. These mutual reinforcement mechanisms create robustness against fluctuations, ensuring stable maintenance of the polarized state throughout critical developmental windows, including the period leading to gastrulation.
Experimental Approaches for Investigating PAR Function in Gastrulation
Genetic Perturbation Strategies
Elucidating PAR protein functions in gastrulation requires precise genetic interventions that disrupt specific components while preserving overall embryonic viability. The following experimental approaches have proven particularly effective:
3.1.1 RNAi-Mediated Gene Knockdown
- Protocol: Feed C. elegans L4-stage larvae with HT115 E. coli expressing dsRNA targeting specific par genes. Transfer P0 animals to fresh RNAi plates after 24 hours and collect embryos from the F1 generation for analysis.
- Key Parameters: dsRNA design should target unique gene regions; include control RNAi against non-essential genes; monitor embryonic lethality and gastrulation defects.
- Applications: This method enables rapid assessment of maternal-effect phenotypes and has been instrumental in establishing the requirements for specific PAR proteins in gastrulation movements [1].
3.1.2 CRISPR/Cas9-Generated Mutants
- Protocol: Inject C. elegans adults with Cas9 protein, gene-specific sgRNAs, and a co-injection marker (e.g., rol-6). Select F1 rollers and establish mutant lines from their progeny. Outcross homozygous mutants to remove off-target mutations.
- Key Parameters: Design sgRNAs to target functional domains; use temperature-sensitive alleles for lethal mutations; employ balancer chromosomes to maintain lethal mutations.
- Applications: Generation of null alleles and precise domain-specific mutations has revealed structure-function relationships for PAR proteins in gastrulation [11].
3.1.3 Auxin-Inducible Degradation System
- Protocol: Express the plant-specific F-box protein TIR1 under tissue-specific promoters in worms carrying AID-tagged PAR proteins. Apply auxin to embryos at specific developmental stages to trigger targeted protein degradation.
- Key Parameters: Titrate auxin concentration (typically 0.5-1.0 mM) to achieve complete degradation without off-target effects; include non-degradable controls; time treatments to specific gastrulation stages.
- Applications: This system enables temporal control of protein function, allowing researchers to define precisely when PAR proteins are required for gastrulation events [11].
Live Imaging and Quantitative Analysis
Visualizing PAR protein dynamics during gastrulation requires high-resolution live imaging coupled with computational analysis:
3.2.1 Fluorescent Tagging of PAR Proteins
- Protocol: Generate transgenic lines expressing endogenously tagged PAR proteins (e.g., PAR-2::GFP, PAR-6::mCherry) using CRISPR/Cas9. For simultaneous visualization of multiple proteins, combine tags with distinct spectral properties.
- Key Parameters: Validate functionality of tagged proteins; minimize tag size to reduce perturbation; confirm expression levels match endogenous patterns.
- Applications: Live tracking of PAR protein localization during gastrulation movements reveals dynamic redistribution in response to cellular rearrangements [12].
3.2.2 Quantitative Image Analysis
- Protocol: Acquire time-lapse images of developing embryos expressing fluorescently tagged PAR proteins. Use computational tools to quantify cortical fluorescence intensity, domain boundaries, and protein redistribution rates.
- Key Parameters: Maintain consistent imaging conditions; correct for photobleaching; normalize fluorescence intensities to cytoplasmic background; analyze multiple embryos (n≥10) for statistical power.
- Applications: This approach has revealed the kinetics of PAR domain establishment in the P1 cell, with posterior PAR-2 domains forming within 4 minutes of division [12].
Visualization of PAR Protein Signaling Networks
Core PAR Protein Interaction Network
PAR Protein Regulatory Network: This diagram illustrates the core interactions between anterior PAR complexes (blue), posterior PAR complexes (red), and CDC-42 (yellow) that establish and maintain cellular asymmetry. The network highlights how mutual exclusion between anterior and posterior PAR domains creates stable polarity, which then directs downstream processes including spindle orientation and cell fate specification—both critical for gastrulation.
Experimental Workflow for PAR Protein Functional Analysis
PAR Protein Experimental Workflow: This workflow outlines the key steps for investigating PAR protein function in gastrulation, from genetic manipulation to phenotypic analysis. The sequential process ensures comprehensive assessment of how polarity disruptions impact morphogenetic movements.
Research Reagent Solutions for PAR Protein Studies
Table 3: Essential Research Tools for Investigating PAR Proteins in Gastrulation
| Reagent Category | Specific Examples | Research Application | Key Features |
|---|---|---|---|
| Genetic Tools | par-2(lt1), par-3(zu310), pkc-3(RNAi) | Loss-of-function studies | Maternal-effect embryonic lethal phenotypes |
| Live Imaging Reagents | PAR-2::GFP, PAR-6::mCherry, PH::GFP | Protein localization and dynamics | Endogenous tagging, minimal perturbation |
| Perturbation Systems | AID::PAR-3, TIR1 expression | Temporal protein degradation | Stage-specific inactivation |
| Cell Biology Probes | Anti-PAR-1 antibody, Rhodamine-phalloidin | Cytoskeletal coordination | F-actin visualization with PAR protein staining |
| Computational Tools | PAR protein domain quantification scripts | Quantitative analysis of polarity | Automated boundary detection, intensity profiling |
The reagents listed in Table 3 represent essential tools for dissecting PAR protein functions during gastrulation. Genetic tools enable researchers to disrupt specific PAR components and assess the functional consequences. Live imaging reagents facilitate direct visualization of protein dynamics throughout the polarization process. Importantly, recent advances in conditional perturbation systems, such as the auxin-inducible degradation (AID) system, allow precise temporal control over protein function, enabling researchers to define precisely when PAR proteins are required for specific gastrulation events [11]. These tools collectively provide a comprehensive toolkit for investigating how PAR-mediated polarity directs morphogenesis.
The PAR protein network represents a fundamental mechanism for translating molecular asymmetries into coordinated cell behaviors during embryogenesis. In C. elegans, PAR proteins not only establish the anterior-posterior axis in the one-cell embryo but also continue to function in descendant cells to direct the cell fate decisions and polarized divisions that enable gastrulation. The mutual inhibition between anterior and posterior PAR complexes, reinforced by feedback loops involving CDC-42 and cytoskeletal networks, creates robust polarity that withstands developmental perturbations. Continued investigation of PAR protein dynamics using the experimental approaches outlined herein will further elucidate how cellular polarity is harnessed to drive the complex tissue rearrangements that characterize gastrulation across metazoans.
Decoding Polarity Dynamics: Biochemical, Biophysical, and Computational Approaches
This technical guide provides a comprehensive framework for applying Fluorescence Recovery After Photobleaching (FRAP) to investigate the dynamics of PAR proteins in living C. elegans embryos. Within the context of gastrulation research, understanding PAR protein dynamics is essential as these conserved regulators establish apical-basal polarity required for proper cell ingression movements. We present detailed methodologies for quantifying PAR protein membrane affinity, diffusion coefficients, and turnover rates, along with analytical approaches for interpreting recovery kinetics within the framework of PAR network interactions. The protocols and data analysis pipelines enable researchers to decipher how balanced antagonism between anterior and posterior PAR complexes patterns embryonic cells for asymmetric division and morphogenetic events during gastrulation.
PAR Protein Fundamentals
The partitioning-defective (PAR) proteins form an evolutionarily conserved system that establishes cellular polarity across animal species. Initially discovered in genetic screens for regulators of cytoplasmic partitioning in the early C. elegans embryo [1], the six core PAR proteins organize into two functionally antagonistic groups: the anterior complex (PAR-3, PAR-6, and aPKC) and the posterior complex (PAR-1, PAR-2, and PAR-5/14-3-3) [1] [10]. These proteins segregate into mutually exclusive cortical domains, creating a fundamental polarity axis that directs asymmetric cell division and cell fate determination.
During C. elegans gastrulation, which begins at the 26-cell stage, PAR proteins undergo a remarkable transition from anterior-posterior polarization in the one-cell embryo to apical-basal polarization in somatic cells [14]. PAR-3, PAR-6, and PKC-3 become enriched on apical surfaces, while PAR-1 and PAR-2 localize to basolateral surfaces [14]. This apical-basal polarization is essential for proper blastocoel formation and guides the ingression movements of endodermal precursors (Ea and Ep) and other cells during gastrulation [14]. The PAR network thus provides the structural and signaling framework that enables gastrulation movements by regulating cell adhesion properties and actomyosin contractility.
Significance of PAR Protein Dynamics
PAR domains maintain remarkable stability despite constant molecular turnover, suggesting that these systems exist in a dynamic steady state rather than a static configuration. Understanding how polarity is maintained requires quantitative analysis of PAR protein kinetics, including their membrane association/dissociation rates, lateral mobility, and response to perturbation. FRAP has emerged as a powerful method for quantifying these dynamics in living embryos, revealing that PAR proteins undergo continuous exchange between cytoplasmic and membrane-associated states while maintaining sharp domain boundaries [4].
The balance between antagonizing PAR complexes not only specifies asymmetric division patterns but also regulates the transition to symmetric divisions during embryonic development [10]. Quantitative measurements of PAR dynamics are therefore essential for understanding how embryonic cells interpret and remodel polarity information during gastrulation and subsequent morphogenetic events.
FRAP Fundamentals and Experimental Design
Principles of FRAP
Fluorescence Recovery After Photobleaching (FRAP) is a powerful method to investigate the dynamics of molecules in living cells [15]. In a FRAP experiment, fluorescent molecules in a defined region are irreversibly photobleached using a high-power laser, and the subsequent recovery of fluorescence into the bleached area is monitored over time [15]. The recovery kinetics provide quantitative information about molecular mobility, binding interactions, and transport mechanisms.
For membrane-associated proteins like PAR components, FRAP can distinguish between several potential mobility mechanisms:
- Lateral diffusion: Movement along the membrane plane
- Cytoplasmic exchange: Rapid association/dissociation with the membrane
- Directed transport: Active movement via motor proteins or flow
The spatial pattern of recovery is particularly informative: lateral diffusion produces faster recovery at the edges of the bleached region, while pure cytoplasmic exchange results in spatially uniform recovery [4].
PAR-Specific FRAP Considerations
When designing FRAP experiments for PAR proteins, several specialized considerations apply:
Developmental Timing: PAR protein dynamics differ significantly between polarity establishment and maintenance phases. The maintenance phase (after the PAR domains have formed) is particularly suitable for quantitative measurements due to the absence of large-scale cortical flows and relative stability of domain boundaries [4].
Genetic Background: To isolate the intrinsic behavior of individual PAR proteins, experiments may be performed in embryos depleted of opposing PAR factors (e.g., analyzing GFP-PAR-6 in PAR-2 depleted embryos) [4]. This eliminates the confounding effects of mutual antagonism during recovery.
Spatial Positioning: The location of bleaching regions relative to domain boundaries provides information about potential diffusion barriers and directional biases in recovery.
Table 1: Key Experimental Parameters for PAR Protein FRAP
| Parameter | Consideration | Typical Setting for PAR Proteins |
|---|---|---|
| Bleaching Region | Size and shape | Circular spot, 7-pixel radius [15] |
| Background Regions | Reference for bleaching correction | Non-bleached area in same domain [15] |
| Temporal Resolution | Balance between kinetics and phototoxicity | 0.242 sec/cycle for PAR-2/PAR-6 [4] |
| Laser Power | Sufficient bleaching without damage | 50% transmission, 20 iterations [15] |
| Developmental Stage | Maintenance phase preferred | After polarity establishment, before gastrulation |
Experimental Protocols for C. elegans Embryos
Sample Preparation and Mounting
C. elegans Strains and Transgenes
- Use transgenic strains expressing functional GFP-tagged PAR proteins (e.g., GFP::PAR-6 or GFP::PAR-2) that complement null mutations [4]
- Maintain transgenes in appropriate genetic backgrounds; for extrachromosomal arrays, select GFP-positive worms for experiments [15]
- For membrane fluidity studies, utilize strains expressing membrane-targeted FPs (e.g.,
glo-1p::GFP::ras-2 CAAXfor intestinal plasma membranes) [15]
Synchronization and Preparation
- Bleach gravid hermaphrodites to obtain synchronized eggs [15]
- Allow eggs to hatch overnight in M9 buffer to collect synchronized L1 larvae [15]
- Transfer L1 larvae to NGM plates seeded with OP50 E. coli, potentially supplemented with experimental compounds (e.g., 20 mM glucose for membrane rigidity studies) [15]
- Incubate at 20°C for 16 hours until worms reach desired developmental stage (L2 for intestinal studies) [15]
Embryo Mounting
- Prepare 2% agarose pads on microscope slides [15]
- Transfer transgenic worms (20-25) to agarose pad in 1-1.5 μL of 100 mM levamisole to paralyze without complete immobilization [15]
- Apply cover glass gently to avoid compression artifacts
- Verify embryo viability and developmental stage before imaging
Microscope Configuration
Hardware Requirements
- Confocal microscope with 40x water immersion objective (Numerical Aperture 1.1 recommended) [15]
- 488 nm laser for GFP excitation [15]
- Temperature control to maintain embryos at 20-25°C during imaging
Software Settings
- Use frame mode with 12-bit intensity resolution over 256 × 256 pixels [15]
- Set digital zoom to 4x and pixel dwell time to 1.58 μsec [15]
- Use unidirectional scanning with pinhole set to 1 Airy Unit [15]
- Configure time series with 200 cycles at 242.04 msec/cycle [15]
- Set up bleaching parameters: 20 iterations at 50% laser power transmission [15]
- Adjust master gain and digital offset to avoid signal saturation [15]
FRAP Execution Protocol
- Localization: Using bright field or low-intensity fluorescence, identify a suitable embryo with clear PAR protein localization
- Baseline Acquisition: Acquire 10 pre-bleach scans to establish baseline fluorescence [15]
- Photobleaching: Bleach a circular region (7-pixel radius) using high-power laser settings [15]
- Recovery Monitoring: Continuously scan the region for 200 cycles to monitor fluorescence recovery [15]
- Data Export: Save both images and fluorescence intensity tables (.txt format) for subsequent analysis [15]
Quantitative Analysis of FRAP Data
Data Preprocessing
FRAP recovery curves require three correction steps before quantitative analysis:
1. Background Subtraction
- Subtract background intensity from non-cellular regions
- Apply to all frames in the time series
2. Bleaching Correction
- Select a reference region in the non-bleached area of the same domain [15]
- Calculate the slope of fluorescence decrease in the reference region
- Compensate for global bleaching during acquisition: divide each FRAP measurement by [1 - (bleaching_rate × time)] [15]
3. Normalization
- Identify the minimum intensity value immediately after bleaching and subtract from all values [15]
- Calculate average intensity of 5 pre-bleach measurements and divide all intensities by this value [15]
- Express recovery as normalized intensity from 0 (immediately post-bleach) to 1 (full recovery)
Kinetic Parameter Extraction
The corrected recovery curve can be fit to appropriate models to extract quantitative parameters:
Mobile Fraction (Mf)
- Mf = (I∞ - I0) / (Ii - I0)
- Where I∞ is plateau intensity, I0 is immediate post-bleach intensity, Ii is initial pre-bleach intensity
- Represents the proportion of molecules free to exchange during the experimental timeframe
Half-Time of Recovery (tâ‚/â‚‚)
- Time required to reach half of maximum recovery
- Inversely related to exchange kinetics
Diffusion Coefficient (D)
- For lateral diffusion, D can be estimated from recovery half-time: D = 0.224 × r² / tâ‚/â‚‚
- Where r is the radius of the bleached spot
Table 2: Quantitative FRAP Parameters for PAR Proteins
| PAR Protein | Mobile Fraction | Recovery Half-Time (tâ‚/â‚‚) | Diffusion Coefficient | Experimental Conditions |
|---|---|---|---|---|
| PAR-6 | High (~80-90%) | Rapid (~seconds) | ~0.1 μm²/s | PAR-2 depleted embryos [4] |
| PAR-2 | High (~80-90%) | Rapid (~seconds) | ~0.1 μm²/s | PAR-6 depleted embryos [4] |
| Membrane Marker | Variable | Dependent on fluidity | Environment-dependent | Varies with lipid composition [15] |
Spatial Analysis of Recovery Patterns
The spatial characteristics of FRAP recovery provide critical information about mobility mechanisms:
Edge-Enhanced Recovery: Indicates significant lateral diffusion component, as molecules diffuse into the bleached area from adjacent regions [4]
Uniform Recovery: Suggests exchange-dominated kinetics, with molecules arriving from the cytoplasm rather than adjacent membrane regions
For PAR proteins, both PAR-6 and PAR-2 demonstrate edge-enhanced recovery, indicating substantial lateral diffusion along the membrane plane [4]. This spatial signature is consistent with free diffusion of molecules across domain boundaries, countered by spatially varying membrane affinities rather than diffusion barriers.
PAR Protein Dynamics in Gastrulation Context
Apical-Basal Polarization
During gastrulation, PAR proteins transition from anterior-posterior polarization to apical-basal polarization in somatic cells [14]. By the end of the four-cell stage, PAR-3 becomes restricted to apical surfaces while PAR-2 localizes to basolateral surfaces [14]. This apical-basal asymmetry depends on cell contacts and directs the pattern of cell adhesions that form the blastocoel cavity [14].
FRAP analysis reveals that PAR proteins maintain dynamic exchange even while stably localized to specific membrane domains. This dynamic steady state enables cells to remodel polarity during gastrulation as they change shape, position, and contacts.
Regulation of Cell Ingression
PAR proteins directly regulate the actomyosin dynamics that drive cell ingression during gastrulation. The endodermal precursors Ea and Ep accumulate non-muscle myosin NMY-2 at their apical surfaces as they ingress [14]. PAR proteins localized to apical surfaces are required for this apical accumulation of myosin [14].
The balance between anterior and posterior PAR complexes determines division mode (asymmetric vs. symmetric) during development [10]. Changes in the PAR-2/PAR-6 balance can reprogram division modes independently of other asymmetries [10], highlighting how PAR protein dynamics directly influence cell behavior during gastrulation.
Diagram 1: PAR Protein Network in Gastrulation Context. PAR proteins establish both anterior-posterior and apical-basal polarity through mutual antagonism, then regulate gastrulation processes including myosin localization and cell adhesion.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for PAR Protein FRAP Studies
| Reagent/Condition | Function/Application | Example Use in PAR Studies |
|---|---|---|
| GFP-tagged PAR strains | Functional fusions for live imaging | QC114 for membrane dynamics; endogenous CRISPR-tagged PAR proteins [15] [16] |
| par-2(RNAi) | Deplete posterior PAR domain | Study PAR-6 dynamics without antagonism [4] |
| par-6(RNAi) | Deplete anterior PAR domain | Study PAR-2 dynamics without antagonism [4] |
| Levamisole (100 mM) | Reversible immobilization | Paralyze embryos without fixation [15] |
| Agarose pads (2%) | Physiological mounting substrate | Support embryos during imaging [15] |
| NMY-2::GFP | Monitor actomyosin dynamics | Visualize apical constriction during ingression [14] |
| FRAP configuration | Standardized bleaching protocol | 7-pixel radius, 20 iterations, 50% laser power [15] |
| Temperature control | Maintain physiological conditions | 20°C during imaging for normal development |
| Cefmatilen | Cefmatilen, CAS:140128-74-1, MF:C15H14N8O5S4, MW:514.6 g/mol | Chemical Reagent |
| LY 121019 | Cilofungin CAS 79404-91-4|For Research | Cilofungin is a first-generation echinocandin antifungal agent for research. It inhibits β-(1,3)-D-glucan synthase. This product is For Research Use Only. |
Signaling Pathways and Molecular Interactions
The PAR protein network operates through a system of mutual antagonism and spatially regulated kinase-phosphatase activities:
Anterior Complex Signaling
- PAR-3, PAR-6, and aPKC form a stable complex [1]
- CDC-42 binds PAR-6 and promotes cortical association [1]
- aPKC phosphorylates posterior PAR proteins to exclude them from the anterior domain
Posterior Complex Signaling
- PAR-1 and PAR-2 form a mutually reinforcing complex [1]
- PAR-1 phosphorylates anterior components to exclude them from the posterior
- PAR-5/14-3-3 facilitates mutual exclusion by binding phosphorylated residues [1]
Integration with Gastrulation Machinery
- PAR proteins regulate apical accumulation of non-muscle myosin II [14]
- Myosin contraction drives apical constriction of ingressing cells
- PAR-dependent adhesion patterning controls blastocoel formation [14]
Diagram 2: PAR Protein Signaling Network. Anterior and posterior PAR complexes mutually exclude each other through phosphorylation events, then regulate effectors for gastrulation including myosin and adhesion proteins.
Advanced Applications and Future Directions
Integration with Other Biophysical Techniques
FRAP data becomes more powerful when combined with complementary approaches:
FLIP (Fluorescence Loss in Photobleaching): Assesses intercompartmental connectivity by repeatedly bleaching an area and monitoring fluorescence loss in adjacent regions
FCS (Fluorescence Correlation Spectroscopy): Measures diffusion coefficients and concentrations at very small spatial scales
FRET (Förster Resonance Energy Transfer): Probes molecular interactions and conformational changes in living embryos
Computational Modeling Approaches
Quantitative FRAP data enables computational modeling of PAR network dynamics:
Reaction-Diffusion Models: Test whether proposed interaction networks can generate and maintain polarized states
Stochastic Simulations: Account for low copy numbers of some PAR components and potential noise in the system
Parameter Optimization: Use FRAP recovery curves to constrain unknown kinetic parameters in mathematical models
Future Technical Developments
Emerging technologies will enhance PAR protein dynamics studies:
Improved FP Variants: Brighter, more photostable fluorescent proteins (e.g., mNeonGreen, mScarlet) enable longer imaging with reduced phototoxicity [16]
CRISPR/Cas9 Genome Editing: Precise endogenous tagging eliminates artifacts from overexpression and ensures proper regulation [16]
Light Sheet Microscopy: Reduces photobleaching and enables long-term 3D imaging of PAR dynamics during entire gastrulation process
Super-Resolution Techniques: Reveal nanoscale organization of PAR domains beyond diffraction limit
FRAP provides a powerful quantitative approach for investigating PAR protein dynamics in living C. elegans embryos. The methodologies outlined in this guide enable researchers to measure key kinetic parameters that govern the establishment and maintenance of cellular polarity during gastrulation. The dynamic nature of PAR proteins, with continuous exchange between membrane and cytoplasmic pools coupled with free lateral diffusion, reveals that polarity maintenance is an active process requiring balanced antagonism rather than a static distribution. As technical capabilities advance, integrating FRAP with complementary approaches will further elucidate how PAR protein dynamics regulate the cell behaviors that drive gastrulation and embryonic morphogenesis.
Cell polarization, the process by which cells establish spatial asymmetry, is a fundamental biological phenomenon governing critical processes including embryonic development, cell division, and cell fate specification. In the nematode Caenorhabditis elegans embryo, this polarization is orchestrated by partitioning-defective (PAR) proteins, which form opposing domains on the cell membrane and control asymmetric cell divisions [17] [18]. These divisions are crucial for patterned tissue growth and cell fate specification during gastrulation, the complex morphological rearrangement where embryonic cells form the three germ layers [11]. Understanding the role of PAR proteins in gastrulation requires uncovering how their spatiotemporal dynamics influence downstream cell behaviors. Reaction-diffusion modeling provides a powerful computational framework to simulate these dynamics, offering insights into how biochemical networks and physical constraints interact to generate robust polarization patterns in realistic cellular geometries. This technical guide explores current methodologies for simulating PAR network behavior, with emphasis on applications to gastrulation research in C. elegans.
Biological Foundation: The PAR Protein Network
Core PAR Protein Components and Interactions
The core PAR polarization system in the C. elegans zygote consists of two antagonistic groups localized to opposite membrane domains. The anterior complex includes PAR-3, PAR-6, and atypical protein kinase C (PKC-3), while the posterior complex comprises PAR-1 and PAR-2 [17] [18]. Following fertilization, the sperm entry point defines the posterior pole, triggering a contraction of cortical actomyosin that excludes the anterior PAR complex from the posterior region, allowing the posterior PAR complex to establish its domain [18]. During the maintenance phase, these two groups engage in mutual inhibition: membrane-associated PAR-3/PAR-6/PKC-3 inhibits the recruitment of PAR-1/PAR-2, and vice versa [17]. This reciprocal exclusion forms the backbone of a robust reaction-diffusion system that maintains stable polarized states.
Expanded Network Complexity
Recent research has identified additional proteins that significantly interact with the core PAR network, increasing its complexity and robustness. Key players include CDC-42, LGL-1, and CHIN-1, which modify the network's dynamics during the maintenance phase [17] [18]. These components introduce additional regulatory pathways, such as mutual activation in the anterior and additional mutual inhibition between anterior and posterior domains [18]. This expanded connectivity forms a 5-node network that enhances stability and enables precise control over the boundary position between anterior and posterior domains, crucial for proper asymmetric cell divisions during gastrulation.
Table 1: Core PAR Protein Complexes in C. elegans
| Domain | Protein Components | Key Functions |
|---|---|---|
| Anterior | PAR-3, PAR-6, PKC-3 | Forms apical caps; inhibits posterior complex; orient mitotic spindle [11] |
| Posterior | PAR-1, PAR-2 | Excludes anterior complex; regulates spindle orientation [11] |
| Regulatory | CDC-42, LGL-1, CHIN-1 | Modifies network stability and asymmetry; provides robustness [18] |
Computational Modeling Approaches
Reaction-Diffusion Principles for PAR Networks
Reaction-diffusion models describe how the concentrations of PAR protein complexes evolve in space and time through the combined effects of chemical reactions (association/dissociation, mutual inhibition) and spatial diffusion. The dynamics of each molecular species ( X ) can be captured through conservation equations that account for its membrane association rate ( F{on}^X(x,t) ), dissociation rate ( F{off}^X(x,t) ), and diffusion along the membrane and in the cytosol [18]. The mutual inhibition between anterior and posterior PAR complexes creates a nonlinear feedback that enables pattern formation, while the significant difference in diffusion rates between cytosolic and membrane-bound states (approximately two orders of magnitude higher in the cytosol) contributes to the stability of the polarized pattern [18].
Modeling Workflow for Realistic Cell Geometries
Implementing reaction-diffusion models for PAR networks in realistic cell geometries involves several key stages. First, cellular and subcellular geometries must be discretized using appropriate meshing techniques. The Spatial Modeling Algorithms for Reactions and Transport (SMART) software package utilizes tetrahedral meshes derived from microscopy images to accurately represent complex cellular morphologies [19]. Next, reaction-diffusion equations are defined over these computational domains, with careful attention to mixed-dimensional couplings (e.g., bulk-surface reactions at organelle membranes). Finally, numerical solutions are obtained using finite element methods, which provide high accuracy and geometric flexibility while conserving mass and momentum [19].
Diagram 1: Computational Modeling Workflow for PAR Networks. This workflow illustrates the key stages in developing realistic reaction-diffusion models of PAR protein dynamics, from geometry acquisition to results analysis.
Implementing PAR Network Simulations
Network Topologies and Their Properties
The simplest representation of the PAR system is an antagonistic 2-node network comprising mutual inhibition between anterior and posterior complexes. While this minimal model can generate polarization, it exhibits translational symmetry, meaning the boundary between domains can be stabilized at any location [18]. Realistic PAR networks incorporate additional components that break this symmetry and stabilize the boundary at specific positions. Research shows that unbalanced modifications—such as single-sided self-regulation, single-sided additional regulation, or unequal system parameters—can cause polarized patterns to collapse into homogeneous states. However, combining two or more unbalanced modifications with opposing effects can restore pattern stability through fine-tuning of kinetic parameters [18].
Spatial Considerations and Boundary Control
In realistic cell geometries, the PAR network interface can be stabilized at designated locations using spatially inhomogeneous parameters that favor respective domains. This strategy is employed in the C. elegans cell polarization network to maintain pattern stability while controlling interface localization [18]. Computational studies demonstrate that a step-like spatial profile of kinetic parameters, with values leading to opposing velocities when the interface is displaced, can effectively pin the boundary at specific positions. This mechanism enables the robust asymmetry required for proper cell positioning during embryonic development, including gastrulation events [11].
Table 2: Modeling Approaches for PAR Networks
| Approach | Key Features | Applications | Tools/Software |
|---|---|---|---|
| 2-Node Network | Mutual inhibition; minimal model; translational symmetry | Basic pattern formation studies; theoretical analysis | Custom simulations [18] |
| 5-Node Network | Additional regulators (CDC-42, LGL-1); enhanced stability; controlled asymmetry | Realistic C. elegans polarization; gastrulation studies | PolarSim [18] |
| Finite Element Methods | Realistic geometries; accurate spatial resolution; bulk-surface reactions | Subcellular signaling; complex cell morphologies | SMART, FEniCS [19] |
| Agent-Based Monte Carlo | Stochastic dynamics; discrete particles; spatial correlations | Fluctuation effects; small molecule numbers | Custom implementations [20] |
Technical Implementation Guide
Geometry Handling and Meshing Techniques
Realistic reaction-diffusion modeling requires accurate representation of cellular geometries. The GAMer 2 (Geometry-preserving Adaptive Mesher version 2) software enables conversion of microscopy images into well-conditioned tetrahedral meshes suitable for finite element simulations [19]. These meshes can be annotated to mark subcellular structures such as the nucleus, endoplasmic reticulum, or mitochondria, along with their respective membrane boundaries. When implementing PAR network models, special attention should be paid to membrane surfaces where PAR proteins localize, as these 2D manifolds embedded in 3D space require specialized numerical treatment in the form of mixed-dimensional partial differential equations [19].
Mathematical Formulation and Numerical Methods
The reaction-transport dynamics of PAR proteins can be described by systems of mixed-dimensional partial differential equations. For a molecular species ( X ), the general form includes diffusion terms within compartments and reactive fluxes across boundaries:
[ \frac{\partial X}{\partial t} = DX \nabla^2 X + RX({C_j}) ]
where ( DX ) is the diffusion coefficient and ( RX ) represents the net reaction rate depending on concentrations of other species. At membrane boundaries, coupling conditions describe association and dissociation kinetics. The SMART software package implements these equations using finite element discretization, which provides numerical stability and conservation properties essential for accurate long-time simulations [19]. For parameterization, experimental measurements indicate that PAR protein diffusion rates are approximately two orders of magnitude higher in the cytosol compared to membrane-bound states [18].
Research Reagent Solutions
Table 3: Essential Research Reagents and Computational Tools
| Reagent/Tool | Function/Application | Key Features |
|---|---|---|
| PolarSim Software | Exploration of PAR network dynamics | User-friendly interface; customizable node numbers and parameters; simulation of 2-node to 5-node networks [18] |
| SMART Software Package | Solving reaction-diffusion systems in realistic geometries | Finite element method; handles mixed-dimensional PDEs; compatibility with cellular meshes [19] |
| GAMer 2 Meshing Software | Converting microscopy data to computational meshes | Geometry preservation; adaptive meshing; annotation of subcellular structures [19] |
| FEniCS Project | Finite element computation | High-performance solvers; variational form formulation; parallel processing capabilities [19] |
Case Study: PAR Dynamics in Early C. elegans Embryos
Apical PAR Caps and Spindle Orientation
Recent research using spatiotemporally controlled protein degradation and live embryo imaging has revealed that PAR proteins form apical caps at the center of the contact-free membrane in pre-gastrula C. elegans embryos [11]. These PAR-3/aPKC caps localize dynamically during the cell cycle and play a crucial role in orienting the mitotic spindle, which subsequently influences proper cell positioning. Surprisingly, isolated single blastomeres lacking cell contacts can break symmetry autonomously and form PAR-3/aPKC caps independently of actomyosin flows and microtubules [11]. This autonomous polarization capability demonstrates the robustness of the underlying reaction-diffusion network.
Inside-Outside Polarity and Gastrulation
The apical PAR caps establish an "inside-outside" polarity that orients cell divisions in pre-gastrula C. elegans embryos, analogous to radial polarity in other animal embryos [11]. This polarity mechanism contributes to the controlled pattern of cell divisions and positioning that underlies gastrulation movements. Computational models that incorporate these autonomous polarization capabilities can help elucidate how local cell interactions translate into global tissue reorganization during this critical developmental phase.
Diagram 2: PAR Protein Signaling Pathway in C. elegans. This diagram illustrates the core PAR protein signaling pathway from initial symmetry breaking through to gastrulation movements, highlighting key regulatory interactions.
Future Directions and Technical Challenges
Integrating Multiple Spatial and Temporal Scales
A significant challenge in modeling PAR networks lies in integrating multiple spatial and temporal scales. PAR protein interactions occur at molecular scales (nanometers) and milliseconds, while their effects on cell division and gastrulation unfold at cellular scales (micrometers) over minutes to hours. Multi-scale modeling approaches that efficiently bridge these dimensions will be essential for comprehensive understanding of how PAR dynamics influence embryonic development. Recent advances in adaptive meshing and hybrid simulation methods offer promising avenues for addressing these challenges [19].
Parameter Estimation and Model Validation
Accurate parameterization of PAR network models remains difficult due to limited quantitative measurements of kinetic parameters in vivo. Fluorescence recovery after photobleaching (FRAP) and single-molecule imaging provide some constraints, but further experimental quantification is needed [18]. Additionally, model validation requires comparison not only with wild-type phenotypes but also with precise spatiotemporal measurements from perturbation experiments, such as protein degradation or RNA interference. The development of standardized benchmarking datasets for PAR network dynamics would significantly advance the field.
Reaction-diffusion modeling of PAR networks in realistic cell geometries provides a powerful approach for understanding the role of these proteins in C. elegans gastrulation. By combining detailed biochemical networks with accurate geometrical representations, researchers can simulate how autonomous cellular polarization translates into coordinated tissue reorganization. Current computational tools like SMART and PolarSim enable increasingly realistic simulations that capture the essential features of PAR protein dynamics while accommodating the complex morphology of actual cells. As these methods continue to evolve, they will offer deeper insights into the fundamental principles linking subcellular biochemistry to multicellular morphogenesis during embryonic development.
Within the framework of a broader thesis on the role of PAR proteins in C. elegans gastrulation research, this technical guide delineates the fundamental principles whereby cellular geometry instructs robust cell polarization. Polarization, the asymmetric patterning of cellular components, is a prerequisite for asymmetric cell division, cell migration, and morphogenetic events like gastrulation. The PAR (partitioning defective) proteins, discovered in C. elegans, are evolutionarily conserved master regulators of this process. While the biochemical antagonism between anterior and posterior PAR proteins is known to stabilize polarized states, the mechanisms governing the initial selection of the correct polarization axis—specifically, the long anterior-posterior axis in the zygote—have remained elusive. Recent integrative biophysical models reveal that geometric cues, principally the local ratio of membrane surface to cytosolic volume, are critical for initiating pattern formation and ensuring the long axis is selectively stabilized. This guide provides an in-depth analysis of the underlying mechanisms, quantitative data, and experimental methodologies that define how geometric sensing orchestrates robust polarization, providing a foundational model for analogous processes in diverse cell types, including those implicated in disease.
The PAR proteins form an ancient and fundamental mechanism for establishing cellular asymmetry in animal cells. They were first identified in genetic screens in C. elegans for mutants defective in cytoplasmic partitioning during the early embryonic divisions [1]. Six core PAR genes (par-1 to par-6) were discovered, encoding proteins that localize asymmetrically in the one-cell zygote and are required for the asymmetric positioning of the mitotic spindle and the asymmetric distribution of cell fate determinants [1] [21]. The molecular identities of these proteins suggested they constituted a novel intracellular signaling pathway: PAR-1 and PAR-4 are serine/threonine kinases, PAR-5 is a 14-3-3 protein, PAR-3 and PAR-6 contain PDZ domains and act as signaling scaffolds, and PAR-2 contains a RING finger domain [1].
A core principle of the PAR system is mutual antagonism. In the C. elegans zygote, the "anterior PARs" (aPARs: PAR-3, PAR-6, and PKC-3) and "posterior PARs" (pPARs: PAR-1 and PAR-2) form mutually exclusive cortical domains [1] [22]. The aPAR complex occupies the anterior cortex, while the pPARs occupy the posterior. This asymmetry is established upon fertilization, triggered by a signal from the sperm-donated centrosome that induces actomyosin-driven cortical flows, which displace aPARs anteriorly, allowing pPARs to bind the posterior cortex [22]. Following this establishment phase, the system enters a maintenance phase where the polarized domains are stabilized independently of the initial cytoskeletal trigger [22]. The PAR system is not limited to the early zygote; it also regulates polarization in later embryonic stages, including during gastrulation, where it controls apicobasal polarity in epithelial cells and ingression movements of endodermal cells [23] [24].
The Core Signaling Network: PAR Protein Interactions and CDC-42
The robust polarization observed in the C. elegans embryo emerges from a network of biochemical interactions centered on the PAR proteins and the small GTPase CDC-42. This network can be distilled into a core interaction scheme as shown in the diagram below.
Diagram 1: Core PAR-CDC-42 Interaction Network.
The core logic is one of mutual inhibition between the aPAR and pPAR groups:
- The aPAR complex (PAR-3/PAR-6/PKC-3) phosphorylates pPARs (PAR-1, PAR-2), which promotes their dissociation from the membrane into the cytosol [2].
- Conversely, pPARs phosphorylate aPARs (particularly PAR-3), triggering their dissociation from the membrane [2].
- The Rho GTPase CDC-42, a conserved polarity protein, engages in a positive feedback loop with the aPARs. Active, membrane-bound CDC-42 recruits and stabilizes the PAR-6/PKC-3 complex at the membrane, and this complex, in turn, helps to maintain CDC-42 in its active, membrane-bound state [1] [2]. Furthermore, pPARs promote the dissociation of CDC-42 from the membrane, reinforcing the mutual exclusivity of the domains [2].
This network of mutual antagonism and positive feedback is sufficient to explain the stability of polarized states once established. However, a critical unanswered question has been how this biochemical system reliably selects the long axis of the cell for polarization over the short axis.
Geometric Cues as Determinants of Polarity Axis
Theoretical models based solely on mutual antagonism, often simulated in simplified 1D geometries, can stabilize polarity but fail to explain why the anterior-posterior (long) axis is robustly selected in the 3D ellipsoidal geometry of the C. elegans zygote. Recent reaction-diffusion modeling in realistic 3D cell geometry has revealed that geometric cues are central to this axis selection process [25] [22] [26].
The Role of the Membrane-to-Cytosol Volume Ratio
A primary geometric cue is the local surface-to-volume ratio, which varies along the membrane of a non-spherical cell. In a prolate spheroid (the approximate shape of the C. elegans zygote), the ratio of membrane area to adjacent cytosolic volume is highest at the cell poles and lowest at the equator [22] [26]. This ratio directly influences the probability that a cytosolic protein will successfully rebind to the membrane after detachment.
The process is modulated by the phosphorylation-dephosphorylation cycle. When a PAR protein is phosphorylated by its antagonist on the membrane, it detaches into the cytosol in an inactive state. It must be dephosphorylated before it can rebind. The timescale of this dephosphorylation (reactivation) determines a reactivation length—the average distance a protein diffuses in the cytosol before it is reactivated and can reattach to the membrane [22].
- Short Reactivation Length: If dephosphorylation is fast (short reactivation length), detached proteins reactivate near their point of origin and are likely to rebind the membrane quickly. This favors stability of the existing pattern.
- Long Reactivation Length: If dephosphorylation is slow (long reactivation length), proteins diffuse far into the cytosol, becoming homogenized. Rebinding then becomes highly dependent on the local availability of membrane binding sites, which is dictated by the surface-to-volume ratio.
Consequently, regions with a high local surface-to-volume ratio (the poles) are preferential sites for protein rebinding and pattern initiation, as the membrane is more "accessible" to the cytosolic pool [22] [26].
Interface Length Minimization for Long-Axis Stabilization
While the surface-to-volume ratio can initiate pattern formation, a second geometric principle is required to explain the specific selection of the long axis. The key factor is the minimization of the interface length between the aPAR and pPAR domains on the membrane [22] [26].
In a realistic 3D geometry, a polarity pattern aligned with the long axis has a shorter, more compact interface between the two domains compared to a pattern aligned with the short axis. The system evolves to minimize the diffusive fluxes of PAR proteins between the cytosol and the membrane. A shorter interface reduces the region of intense mutual antagonism and protein exchange, leading to a more stable and energetically favorable configuration. Therefore, the long-axis polarization is stabilized because it presents the topology with the minimal possible interface length between the antagonistic domains [26].
Table 1: Key Geometric Parameters and Their Roles in Axis Selection
| Parameter | Description | Role in Axis Selection |
|---|---|---|
| Local Surface-to-Volume Ratio | Ratio of membrane area to adjacent cytosolic volume; highest at cell poles. | Acts as the primary initiation cue, making the poles preferential sites for protein rebinding and domain formation [22]. |
| Reactivation Length (( \ell = \sqrt{D/\lambda} )) | Average diffusion distance of a protein during its dephosphorylation time; depends on cytosolic diffusion coefficient ((D)) and dephosphorylation rate ((\lambda)). | Determines sensitivity to geometry. A long reactivation length enhances the system's ability to sense and respond to global cell shape [22]. |
| Domain Interface Length | The length of the boundary between the aPAR and pPAR domains on the cell membrane. | Determines the final stabilization. The system selects the polarity axis that minimizes this interface length, which is the long axis in an ellipsoid [22] [26]. |
Quantitative Modeling of PAR Protein Dynamics
To formalize the biological understanding and test the role of geometry, a specific, biochemistry-based reaction-diffusion model was developed. This model moves beyond effective parameters to explicitly represent key biomolecular reactions [22].
Model Equations and Specifications
The model simplifies the PAR system into two effective membrane-binding species: aPARs (split into a scaffold protein A1 and a kinase complex A2) and pPARs (as a single species P). The reactions are modeled using mass-action kinetics within a realistic 3D ellipsoidal geometry approximating the C. elegans zygote (semi-major axis (a = 27 \mu m), semi-minor axis (b = 15 \mu m)) [22].
The core dynamics are described by the following equations for membrane-bound species ((Am), (Pm)) and their cytosolic counterparts ((Ac), (Pc)):
On the membrane (( \partial \Omega )): [ \begin{aligned} \frac{\partial Am}{\partial t} &= Dm^A \nabla^2 Am + k{\text{on}}^A Ac - k{\text{off}}^{A}(Pm) Am \ \frac{\partial Pm}{\partial t} &= Dm^P \nabla^2 Pm + k{\text{on}}^P Pc - k{\text{off}}^{P}(Am) Pm \end{aligned} ]
In the cytosol (( \Omega )): [ \begin{aligned} \frac{\partial Ac}{\partial t} &= Dc^A \nabla^2 Ac - k{\text{on}}^A Ac + k{\text{off}}^{A}(Pm) Am + \lambda Ac^* \ \frac{\partial Pc}{\partial t} &= Dc^P \nabla^2 Pc - k{\text{on}}^P Pc + k{\text{off}}^{P}(Am) Pm + \lambda Pc^* \end{aligned} ]
Here, the off-rates ( k{\text{off}}(X) ) are functions of the antagonistic protein concentration, embodying mutual phosphorylation. The terms ( \lambda Ac^* ) and ( \lambda P_c^* ) represent the dephosphorylation (reactivation) of inactive phosphorylated proteins, a critical component of the cycle [22].
Key Quantitative Insights from the Model
The model yielded several critical, testable predictions:
Table 2: Model Parameters and Their Quantitative Impact
| Parameter/Variable | Mathematical Representation | Quantitative Impact on System |
|---|---|---|
| Dephosphorylation Rate | (\lambda) | A slow rate (long reactivation length) is crucial for robust long-axis selection. Increasing (\lambda) shortens the reactivation length, reducing geometry sensing and leading to misplaced or multiple polarity axes [22]. |
| Total Protein Number | (N{\text{total}} = \int (Am + Ac + Pm + P_c) dV) | Acts as a robustness factor. Higher total protein levels increase the system's resilience to fluctuations and facilitate reliable pattern formation across a wider range of other parameters [22]. |
| Cytosolic Diffusion | (D_c) (~ (10 \mu m^2/s)) | Fast cytosolic diffusion is essential for global communication and pattern coordination across the cell, allowing the system to "measure" and respond to the entire cellular geometry [22]. |
| Membrane Diffusion | (D_m) (~ (0.1 \mu m^2/s)) | Slow membrane diffusion helps to stabilize the boundary between the aPAR and pPAR domains once formed, preventing domain mixing and maintaining a sharp interface [22]. |
Experimental Protocols and Validation
The predictions of geometric sensing, derived from theoretical models, are supported and validated by a range of experimental approaches in C. elegans.
Protocol: Investigating PAR Polarity in Perturbed Geometries
Objective: To empirically test the role of cell geometry in PAR axis selection.
- Microtubule Disruption: Treat embryos with microtubule-depolymerizing drugs (e.g., nocodazole). This disrupts the mitotic spindle and can lead to the formation of rounder embryo shapes, altering the inherent long-axis geometry [22].
- Actomyosin Perturbation: Use genetic mutants (e.g., nmy-2) or drugs (e.g., Latrunculin A) to disrupt the actomyosin cortex. This interferes with the establishment-phase flows, uncoupling the initial trigger from the maintenance phase and allowing the intrinsic geometry-sensing capability of the PAR network to be studied in isolation [22].
- Live-Cell Imaging and Quantification: Acquire 3D time-lapse images of the embryo expressing fluorescently tagged PAR proteins (e.g., PAR-2::GFP, PAR-6::mCherry). Quantify the axis of polarity establishment and the stability of the resulting PAR domains in both wild-type and geometrically perturbed embryos.
Expected Outcome: In severely perturbed geometries (e.g., near-spherical cells), the PAR system may still polarize, but the axis selection will be stochastic or follow residual geometric cues, demonstrating that while geometry is a major cue, it is not the sole initiator [22].
Protocol: Validating the Role of CDC-42
Objective: To test the predicted positive feedback loop between CDC-42 and aPARs.
- Acute Protein Depletion: Employ auxin-inducible degradation systems or RNAi in a cell-specific manner to deplete CDC-42, PAR-6, or PKC-3 in the early embryo [24] [2].
- Quantitative Microscopy: Use fluorescence recovery after photobleaching (FRAP) to measure the stability (on/off rates) of the remaining PAR proteins at the cortex after depletion.
- Phenotypic Analysis: Assess the penetrance of polarity defects, including failure to maintain aPAR/pPAR domains, symmetric cell divisions, and subsequent embryonic lethality [2].
Expected Outcome: Depletion of CDC-42 is predicted to lead to a failure in maintaining aPARs at the membrane, resulting in an expansion of the pPAR domain, consistent with its role in reinforcing the anterior identity [2].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for PAR Protein and Cell Polarity Studies
| Reagent / Tool | Function / Utility | Example Use in Research |
|---|---|---|
| PAR Fluorescent Reporters (e.g., PAR-2::GFP) | Live imaging of protein localization and dynamics in real time. | Visualizing the establishment and maintenance of posterior PAR domains in wild-type and mutant embryos [22]. |
| Auxin-Inducible Degradation System | Enables rapid, conditional, and cell-specific protein depletion. | Acutely degrading PKC-3 in specific blastomeres to study its role in spindle orientation without early embryonic lethality [24] [11]. |
| RhoGEF / RhoGAP Mutants (e.g., PAC-1) | Tools to dissect upstream regulators of polarity. | Studying how the RhoGAP PAC-1, recruited to cell contacts by E-cadherin, locally excludes PAR-3/PAR-6 to define apicobasal polarity [24]. |
| Kinase Inhibitors | Pharmacological inhibition of specific kinases (e.g., PAR-1). | Testing the role of PAR-1-mediated phosphorylation of PAR-3 in its dissociation from the membrane [2]. |
| Reaction-Diffusion Modeling (Computational) | In silico testing of biochemical network hypotheses. | Developing the 3D model that identified the surface-to-volume ratio and interface minimization as key to axis selection [22] [26]. |
| CJ-13,610 | CJ-13,610, CAS:179420-17-8, MF:C22H23N3O2S, MW:393.5 g/mol | Chemical Reagent |
| CK-119 | CK-119, CAS:197917-10-5, MF:C21H23ClN4O5, MW:446.9 g/mol | Chemical Reagent |
Implications for Gastrulation and Beyond
The principles of geometry-sensing by the PAR system extend far beyond the one-cell zygote and are directly relevant to gastrulation in C. elegans. During gastrulation, embryonic cells must polarize along their apicobasal axis to facilitate ingression movements and tissue organization. This polarization is induced by cell-cell contacts [23] [24].
The molecular pathway linking cell contact to PAR polarization involves the transmembrane protein HMR-1 (E-cadherin), which recruits the RhoGAP PAC-1 to sites of cell contact. PAC-1 locally inhibits the Rho GTPase CDC-42, thereby excluding the PAR-3/PAR-6/aPKC complex from contact sites and restricting it to the contact-free (apical) surface [24]. This mechanism effectively uses the geometry of cell-cell contact to define the polarization axis, demonstrating that the PAR system is adept at interpreting diverse spatial inputs—from global egg shape to local intercellular adhesion—to orchestrate robust asymmetry.
The following diagram illustrates this contact-dependent pathway.
Diagram 2: Cell-Contact-Induced Apicobasal Polarization.
This technical guide has synthesized evidence establishing that robust cell polarization in C. elegans is not solely a consequence of biochemical circuitry but is fundamentally guided by cellular geometry. The PAR protein network, through the dynamics of phosphorylation-dephosphorylation cycles and cytosolic diffusion, senses the local membrane-to-cytosol ratio to initiate pattern formation and subsequently minimizes the interface length between antagonistic domains to stabilize polarization along the long axis. These principles provide a powerful, generic framework for understanding how cells translate physical shape into biochemical asymmetry, a process critical for development from the first embryonic division to the complex cell rearrangements of gastrulation. The integration of quantitative modeling with targeted experimental validation, as detailed herein, offers a blueprint for future investigations into how cells measure and respond to their physical geometry in health and disease.
Within the framework of investigating the role of PAR proteins in C. elegans gastrulation, this technical guide elucidates the phosphorylation-dephosphorylation cycle as a critical kinetic timer governing cellular polarization. Asymmetric cell division, a process fundamental to gastrulation and embryonic development, is orchestrated by the dynamic, antagonistic interplay of PAR proteins. This whitepaper synthesizes recent research to detail the core mechanism whereby phosphorylation by anterior PAR proteins inhibits membrane binding of posterior PAR proteins, while subsequent dephosphorylation reactivates them, creating a tunable temporal delay essential for robust polarization. We provide a quantitative breakdown of this cycle, detailed experimental methodologies for its investigation, and essential research tools, offering a comprehensive resource for scientists and drug development professionals aiming to target polarity pathways.
The partitioning defective (PAR) proteins form an ancient and conserved network that establishes cellular asymmetry, a prerequisite for processes ranging from asymmetric cell division to gastrulation [1]. Initially discovered in genetic screens for regulators of cytoplasmic partitioning in the early C. elegans embryo, the six core PAR proteins constitute a fundamental mechanism for cell polarization [1]. This polarization is not a static state but a dynamic equilibrium maintained by continuous biochemical reactions, chief among them being the phosphorylation-dephosphorylation cycle. This cycle acts as a kinetic timer, controlling the reactivation and rebinding of PAR proteins after they are displaced from the cell membrane by antagonistic factors. Understanding this timer is crucial, as defects in cell polarity underlie various diseases, and the core machinery is highly conserved in humans [1] [27].
The Core Mechanism: A Phosphorylation-Dephosphorylation Timer
At its heart, the establishment of anterior-posterior polarity in the one-cell C. elegans embryo is driven by the mutual antagonism between anterior PARs (aPARs: PAR-3, PAR-6, and the atypical protein kinase C, PKC-3) and posterior PARs (pPARs: PAR-1 and PAR-2) [28] [22]. The phosphorylation-dephosphorylation cycle is the central timer regulating this process.
The Kinase Reaction: Phosphorylation and Membrane Dissociation
The cycle begins with the aPAR complex (PAR-6/PKC-3) phosphorylating the pPAR protein PAR-2 on the cell membrane [28]. This phosphorylation event inhibits PAR-2's capacity to bind the plasma membrane, triggering its release into the cytosol in a phosphorylated, and thus inactive, state [28] [22]. This reaction is the first half of the timer, initiating a period of inactivity for PAR-2.
The Phosphatase Reaction: Dephosphorylation and Reactivation
The second, crucial half of the timer involves protein phosphatase 1 (PP1), specifically its catalytic subunits GSP-1 and GSP-2 in C. elegans [28]. Recent research has identified that PP1 phosphatases dephosphorylate PAR-2 in the cytosol, reactivating its membrane-binding capability and allowing it to rebind the posterior cortex [28]. This dephosphorylation reaction is not a mere reversal; it introduces a kinetic delay. The time a protein spends in the phosphorylated state before being reactivated determines how far it can diffuse away from the membrane, directly influencing the stability of the polarity domain.
Table 1: Key Protein Components of the Phosphorylation-Dephosphorylation Timer
| Protein | Role in Cycle | Functional Category | Effect on Target |
|---|---|---|---|
| PKC-3 (aPKC) | Phosphorylates PAR-2 | Kinase | Inhibits membrane binding, promotes detachment |
| PP1 (GSP-1/GSP-2) | Dephosphorylates PAR-2 | Phosphatase | Reactivates membrane binding, promotes attachment |
| PAR-2 | Target of PKC-3/PP1; recruits PAR-1 | Scaffold/Substrate | Phosphorylation status dictates membrane localization |
| PAR-1 | Phosphorylates aPARs; stabilized by PAR-2 | Kinase | Promotes dissociation of aPAR complexes from membrane |
The following diagram illustrates the core phosphorylation-dephosphorylation cycle that governs PAR-2 dynamics:
Diagram 1: The PAR-2 Phosphorylation-Dephosphorylation Cycle. This timer controls the membrane association of the posterior PAR protein PAR-2 through the opposing actions of the anterior kinase PKC-3 and the ubiquitous phosphatase PP1.
Integration with Gastrulation Context
While the one-cell embryo is a paradigm, the principles of this cycle extend directly to later developmental stages like gastrulation. PAR proteins are known to polarize cells during C. elegans gastrulation, and the same machinery is adapted to regulate polarity in diverse contexts, including epithelial apical-basal polarity and cell migration [1]. The phosphorylation-dephosphorylation timer provides a versatile and evolutionarily conserved mechanism for ensuring that polarization is both inducible and robustly maintained, properties essential for the complex cell rearrangements of gastrulation.
Quantitative Kinetics and Geometric Sensing
The kinetics of the phosphorylation-dephosphorylation cycle are not merely a switch but a finely tuned timer that allows the cell to sense its geometry and polarize along the correct axis.
The Reactivation Length and Rebinding Probability
A key quantitative concept is the reactivation length [22]. This is the average distance a protein (like phosphorylated PAR-2) diffuses in the cytosol after detachment and before being reactivated by dephosphorylation. The reactivation length is calculated as ( L = \sqrt{D \cdot \tau} ), where ( D ) is the diffusion coefficient and ( \tau ) is the average reactivation time (the inverse of the dephosphorylation rate, ( \lambda )) [22]. A longer reactivation time means a protein diffuses farther, making rebinding to the membrane less likely in regions where the local ratio of membrane surface to cytosolic volume is low (e.g., the mid-cell). Conversely, at the cell poles, where this ratio is high, rebinding is more probable.
Role in Long-Axis Polarization
This geometry-sensing capability is critical for the robust selection of the long anterior-posterior axis for polarization. Mathematical modeling in realistic 3D cell geometry has shown that without the kinetic delay introduced by the dephosphorylation cycle, the PAR system would preferentially form patterns along the short axis [22]. The delay ensures that interface between aPAR and pPAR domains, where mutual detachment is highest, is most stable along the long axis, as this minimizes the diffusive fluxes of proteins between the cytosol and membrane [22].
Table 2: Key Quantitative Parameters of the Phosphorylation-Dephosphorylation Timer
| Parameter | Description | Impact on Polarity | Theoretical/Experimental Basis |
|---|---|---|---|
| Dephosphorylation Rate (λ) | Rate constant for PP1-mediated reactivation of PAR-2. | Higher λ = shorter timer = more robust polarity. | Mass-action kinetics in reaction-diffusion models [22]. |
| Reactivation Length (L) | Mean diffusion distance of a protein before reactivation. ( L = \sqrt{D / \lambda} ) | Shorter L increases rebinding probability at poles. | Quantitative analysis of cytosolic gradients [22]. |
| Membrane-to-Volume Ratio | Local ratio of available membrane surface to cytosolic volume. | Higher at poles, promoting protein rebinding. | Geometric analysis of prolate spheroid embryo [22]. |
| Phosphorylation Rate | Rate constant for PKC-3-mediated inhibition of PAR-2. | Determines sharpness of the aPAR-pPAR boundary. | In vitro and in vivo kinase assays [28]. |
Experimental Protocols for Investigating the Cycle
To study this kinetic timer, researchers employ a combination of genetic, biochemical, and cell biological techniques in C. elegans. Below are detailed methodologies for key experiments.
Protocol: Validating the PP1-PAR-2 Interaction
Objective: To confirm the physical interaction between PP1 (GSP-2) and PAR-2 and identify the specific docking motif.
Yeast Two-Hybrid Screening:
- Clone the N-terminal region of the par-2 gene into a yeast two-hybrid bait vector.
- Clone the gsp-2 gene into a prey vector.
- Co-transform both plasmids into a suitable yeast reporter strain and plate on selective media lacking leucine and tryptophan.
- Screen for interaction by assessing growth on media lacking histidine and by performing a quantitative β-galactosidase assay. This identified a degenerate PP1-docking motif, RLFF, in PAR-2 [28].
In Vivo Validation via Site-Directed Mutagenesis:
- Generate a transgenic worm strain expressing a GFP-tagged PAR-2 protein where the RLFF motif is mutated to RAFA (e.g., using CRISPR/Cas9).
- Image live embryos and quantify the cortical localization of GFP::PAR-2(RAFA) compared to wild-type GFP::PAR-2. The mutant protein fails to bind the cortex, mimicking the polarity defects observed upon gsp-1/gsp-2 RNAi depletion [28].
Protocol: Assessing the Functional Outcome in Embryos
Objective: To determine the functional consequences of disrupting the phosphorylation-dephosphorylation timer on embryonic polarity.
RNAi Depletion of Phosphatases:
- Feed adult hermaphrodites bacteria expressing double-stranded RNA (dsRNA) targeting gsp-1 and/or gsp-2 [28]. Control worms are fed bacteria with an empty vector.
- After 24-48 hours, mount the subsequent embryos for live imaging.
Live Imaging and Phenotypic Scoring:
- Acquire time-lapse confocal microscopy images of one-cell embryos expressing fluorescently tagged PAR-2 and aPAR (e.g., PAR-6 or PKC-3).
- Quantify the following polarity phenotypes:
- PAR-2 Mislocalization: Measure the fraction of the cortex occupied by PAR-2. Co-depletion of gsp-1 and gsp-2 leads to PAR-2 being almost entirely cytoplasmic [28].
- Cytokinetic Furrow Position: Measure the position of the first cleavage furrow. In pkc-3 mutants, furrow positioning is defective but is restored by simultaneous gsp-2 depletion [28].
- Division Asynchrony: Record the timing of the divisions of the AB and P1 daughter cells. In wild-type, they divide asynchronously; this is lost in polarized mutants but restored in the pkc-3; gsp-2(RNAi) strain [28].
The following workflow summarizes the genetic and imaging strategies used to dissect this pathway:
Diagram 2: Experimental Workflow for Dissecting the Phosphorylation-Dephosphorylation Timer. The pathway is interrogated through specific genetic perturbations, followed by quantitative live imaging and computational modeling.
The Scientist's Toolkit: Essential Research Reagents
The following table compiles key reagents used in the featured studies to investigate the PAR protein phosphorylation-dephosphorylation cycle. These tools are essential for replicating and extending research in this field.
Table 3: Research Reagent Solutions for PAR Protein Cycle Studies
| Reagent / Tool | Function / Description | Example Use Case |
|---|---|---|
| Auxin-Inducible Degron (AID) | Enables rapid, targeted protein degradation upon auxin application. | Post-embryonic analysis of PAR-6 and PKC-3 function in larval epithelia without embryonic lethality [29]. |
| CRISPR/Cas9 Genome Editing | Precise insertion of tags or mutations into endogenous loci. | Creating endogenously tagged PAR-2 (GFP) or introducing point mutations in the PP1-docking motif (par-2 RAFA) [28] [29]. |
| RNAi Feeding Libraries | Genome-wide libraries of bacterial strains for RNAi by feeding. | Large-scale screens for modifiers of PAR polarity phenotypes; targeted depletion of phosphatases like gsp-1/gsp-2 [27] [28]. |
| Temperature-Sensitive Alleles (ts) | Allows conditional disruption of gene function at restrictive temperatures. | Studying essential genes like pkc-3; used to reveal genetic interactions with gsp-2 [28]. |
| Yeast Two-Hybrid System | Identifies protein-protein interactions in a high-throughput manner. | Initially identifying the physical interaction between GSP-2 (PP1) and the N-terminus of PAR-2 [28]. |
| CM-39 | CM-39, MF:C19H15FN4OS, MW:366.4 g/mol | Chemical Reagent |
The phosphorylation-dephosphorylation cycle emerges as a fundamental kinetic timer that rigorously controls the dynamics of PAR proteins in C. elegans. By introducing a tunable delay between membrane detachment and reactivation, this cycle ensures the robust establishment and maintenance of cell polarity, a process directly relevant to gastrulation and overall embryonic development. The integrated efforts of quantitative live-cell imaging, genetic perturbation, and mathematical modeling have revealed how this biochemical timer interacts with cellular geometry to dictate the axis of polarization. For researchers and drug development professionals, the components of this cycle—particularly the kinases and phosphatases—represent conserved targets whose manipulation could influence cell polarity in health and disease.
Resolving Experimental Challenges in PAR Protein and Gastrulation Studies
This technical guide provides a comprehensive framework for distinguishing between establishment and maintenance defects in cell polarity, focusing on the role of PAR proteins in C. elegans gastrulation research. Cell polarity, orchestrated by conserved PAR proteins, constitutes a fundamental process in embryonic development, with distinct establishment and maintenance phases ensuring proper asymmetric cell division and morphogenetic events. Through systematic analysis of mutant phenotypes, quantitative measurements, and targeted experimental approaches, researchers can precisely identify whether polarity defects originate in initial establishment or subsequent maintenance mechanisms. This distinction proves critical for understanding molecular pathways governing gastrulation and for developing targeted therapeutic interventions in developmental disorders.
The PAR (partitioning defective) proteins form an evolutionarily conserved machinery that establishes and maintains cell polarity across diverse animal systems [1]. First identified in genetic screens for regulators of cytoplasmic partitioning in C. elegans embryos, the six core PAR proteins (PAR-1 to PAR-6) function as fundamental organizers of asymmetric cell division [1]. In the context of C. elegans gastrulation, PAR proteins mediate essential apicobasal asymmetries associated with cell adhesion and morphogenetic movements [30]. The PAR network comprises two functionally antagonistic groups: the anterior PAR complex (PAR-3, PAR-6, and PKC-3) localizes to the anterior cortex, while the posterior PAR proteins (PAR-1 and PAR-2) enrich in the posterior cortex, with PAR-4 and PAR-5 functioning throughout the cortex [1] [31].
Cell polarity establishment and maintenance represent temporally distinct processes with unique molecular requirements. During the establishment phase, PAR proteins become asymmetrically localized through a process driven by cortical actomyosin contraction [13]. This initial polarization creates complementary anterior and posterior cortical domains, establishing the anterior-posterior (A/P) axis. The subsequent maintenance phase stabilizes this asymmetric distribution until cytokinesis, preserving distinct molecular identities at opposing cellular poles despite ongoing cellular dynamics [31]. Disruption of either phase produces characteristic mutant phenotypes, though with different developmental consequences.
Table 1: Key PAR Proteins and Their Functions in C. elegans
| Protein | Molecular Identity | Localization | Primary Function in Polarity |
|---|---|---|---|
| PAR-1 | Serine-threonine kinase | Posterior cortex | Phosphorylation-dependent regulation of cortical targets |
| PAR-2 | RING finger domain protein | Posterior cortex | Exclusion of anterior PARs from posterior cortex |
| PAR-3 | PDZ domain scaffold protein | Anterior cortex | Scaffold for anterior PAR complex formation |
| PAR-4 | Serine-threonine kinase | Cortical/cytoplasmic | Kinase activity for polarity establishment |
| PAR-5 | 14-3-3 protein | Cortical/cytoplasmic | Regulation of PAR domain mutual exclusion |
| PAR-6 | PDZ domain scaffold protein | Anterior cortex | Scaffold linking PKC-3 and CDC-42 |
Distinguishing Establishment vs. Maintenance Defects: Conceptual Framework
Defining Characteristics of Establishment Defects
Establishment defects manifest as failures in the initial polarization process, preventing the formation of complementary PAR domains. Embryos with establishment defects typically display symmetric PAR distribution at the one-cell stage, with anterior and posterior PAR proteins intermixed rather than segregated to opposing poles [13]. This disrupted patterning originates from impaired actomyosin-driven cortical flows, which normally transport anterior PAR complexes toward the anterior pole while clearing space for posterior PAR accumulation at the posterior [31]. Consequently, establishment mutants often exhibit complete loss of anterior-posterior asymmetry, affecting downstream processes including asymmetric spindle positioning and cell fate determinant segregation.
Defining Characteristics of Maintenance Defects
Maintenance defects present differently, with embryos establishing initially normal polarity that subsequently deteriorates over time. In maintenance mutants, PAR domains form correctly during the establishment phase but become unstable during later cell cycles, leading to progressive loss of polarization [30]. This manifests as boundary regression between anterior and posterior PAR domains, with proteins from one domain encroaching into territory previously occupied by the opposing complex [31]. For example, in PAR-2 maintenance defects, NMY-2::GFP regresses from the anterior cortex back into the posterior domain after initial restriction [31]. Maintenance defects frequently permit relatively normal early divisions but disrupt later processes such as gastrulation movements and apicobasal polarization [30].
Temporal Signatures for Phenotype Classification
The timing of phenotype manifestation provides the most reliable criterion for distinguishing establishment versus maintenance defects. Establishment defects become apparent during the first cell cycle, while maintenance defects emerge after apparently normal initial polarization. Temporal degradation approaches that selectively disrupt PAR protein function after the one-cell stage have proven invaluable for this distinction, as they permit normal establishment while specifically compromising maintenance mechanisms [30].
Table 2: Diagnostic Features of Establishment vs. Maintenance Defects
| Feature | Establishment Defects | Maintenance Defects |
|---|---|---|
| Initial PAR localization | Symmetric or incomplete asymmetry | Normal asymmetric localization |
| Temporal onset | First cell cycle | After establishment phase |
| Cortical flows | Disrupted or absent | Initially normal, then destabilized |
| Domain boundaries | Fail to form | Form initially but regress |
| Downstream asymmetries | Severely impaired from beginning | Initially normal, later disrupted |
| Gastrulation defects | Often profound | Variable, cell type-dependent |
Experimental Approaches for Distinguishing Polarity Defects
Temporal Control of Protein Function
Inducible degradation systems provide precise temporal resolution for analyzing PAR protein requirements. The auxin-inducible degradation (AID) system enables targeted depletion of AID-tagged PAR proteins at specific developmental timepoints [29]. This approach involves CRISPR/Cas9-mediated insertion of AID degron sequences into endogenous PAR loci, followed by TIR1 expression to trigger degradation upon auxin application. For example, PAR-6::AID and PKC-3::AID embryos exposed to auxin after the one-cell stage develop maintenance-specific defects, revealing requirements for these proteins in stabilizing established polarity [29].
Hybrid PAR degradation technologies offer complementary approaches, employing temperature-sensitive mutants or light-inducible degradation domains. These techniques enable researchers to selectively disrupt PAR function during either establishment or maintenance phases, precisely mapping temporal requirements [30]. When applying these methods, include appropriate controls for degradation efficiency and specificity, and validate phenotypes with multiple independent degradation systems where possible.
Quantitative Live Imaging and Analysis
Live imaging of fluorescently tagged PAR proteins and downstream effectors provides dynamic readouts of polarity dynamics. Essential parameters include:
- Cortical intensity measurements: Quantify fluorescence intensity of PAR::GFP fusions along the anterior-posterior axis over time
- Domain boundary sharpness: Calculate the steepness of fluorescence transitions between anterior and posterior domains
- Cortical flow velocities: Track movement of PAR protein complexes during establishment phase
- Myosin dynamics: Monitor NMY-2::GFP localization and flow patterns [31]
For reliable quantification, acquire images at consistent temporal resolution (typically 10-30 second intervals for early embryos) and normalize fluorescence intensities to cytoplasmic background. Computational modeling approaches can further extract kinetic parameters from live imaging data, enabling quantitative comparison between wild-type and mutant embryos [13].
Functional Rescue at Different Timepoints
Staged rescue experiments can pinpoint when PAR protein function becomes critical. Using temperature-sensitive mutants or inducible expression systems, researchers can restore PAR function at specific developmental timepoints and assess whether polarity establishment or maintenance is rescued. For example, shifting par-2(ts) embryos to permissive temperature after the establishment phase reveals whether PAR-2 requirement extends into maintenance [31]. Successful rescue after normal establishment indicates maintenance function, while failure to rescue suggests essential establishment roles.
PAR Proteins in C. elegans Gastrulation: Establishment and Maintenance Functions
Gastrulation Defects in PAR Mutants
PAR proteins play essential roles in gastrulation by regulating apicobasal polarization and cell adhesion. Embryos with PAR-3 and PAR-6 defects display characteristic gastrulation abnormalities including ectopic separations between lateral cell surfaces and impaired ingression of mesodermal precursors [30]. These defects reflect failures in establishing or maintaining apicobasal asymmetries required for proper morphogenetic movements. Specifically, PAR-3 and PAR-6 are required for normal accumulation of nonmuscle myosin at apical surfaces of ingressing cells, a process essential for efficient gastrulation movements [30].
Distinguishing Polarity Phases in Gastrulation
In gastrulation contexts, establishment defects manifest as failure to initiate proper apicobasal polarization in epithelial cells, while maintenance defects appear as breakdown of initially established polarity during morphogenetic movements. PAR-3 and PAR-6 function in both anterior-posterior and apicobasal asymmetry, with post-embryonic requirements extending to epidermal polarization and microtubule organization [29]. The use of temporal control methods has been particularly revealing for gastrulation studies, demonstrating that PAR proteins have continuing functions beyond initial axis specification.
Table 3: PAR Protein Mutant Phenotypes in C. elegans Gastrulation
| PAR Gene | Gastrulation Defects | Primary Phase Affected | Molecular Consequences |
|---|---|---|---|
| par-3 | Ectopic lateral separations; slowed ingression | Both establishment and maintenance | Disrupted apical myosin accumulation; impaired cell adhesion |
| par-6 | Failed apical junction formation; ingression defects | Primarily maintenance (post-establishment) | Loss of NOCA-1 localization; disrupted microtubule organization |
| pkc-3 | Molting defects; seam cell division patterning | Maintenance | Disrupted apical domain identity; junction positioning defects |
| par-1 | Altered cytoplasmic patterning; ingression delays | Establishment | Impaired cortical flow; defective spindle positioning |
| par-2 | Boundary regression; asymmetric division defects | Maintenance | Failed exclusion of anterior PARs from posterior domain |
Quantitative Analysis of Mutant Phenotypes
Key Parameters for Phenotype Classification
Systematic quantification of specific cellular parameters enables objective distinction between establishment and maintenance defects. Essential measurements include:
- Establishment phase duration: Time from fertilization to complete PAR domain separation
- Domain boundary position: Anterior-posterior coordinate of PAR domain interface
- Boundary stability: Coefficient of variation in boundary position during maintenance phase
- Cortical fluorescence ratio: Anterior versus posterior PAR protein levels
- Myosin distribution: NMY-2 enrichment at anterior cortex or apical surfaces [31]
These parameters should be measured in multiple embryos (typically n≥10) across at least two biological replicates to ensure statistical robustness. Computational image analysis pipelines can automate these measurements, reducing subjective bias in phenotype classification.
Mathematical Modeling of PAR Dynamics
Mathematical models provide quantitative frameworks for interpreting mutant phenotypes. Reaction-diffusion models incorporating actomyosin dynamics can simulate PAR domain establishment and maintenance, generating testable predictions for how specific mutations affect polarity [13]. These models typically treat anterior and posterior PAR proteins as interacting species whose localization depends on cortical flows and mutual inhibition. By fitting model parameters to experimental data, researchers can determine whether specific mutations primarily affect establishment kinetics or maintenance stability.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for Analyzing PAR Protein Mutant Phenotypes
| Reagent/Tool | Function/Application | Key Utility in Phenotype Distinction |
|---|---|---|
| Auxin-inducible degradation (AID) system | Targeted protein degradation at specific developmental timepoints | Temporal control of PAR protein function to separate establishment vs maintenance requirements |
| PAR::GFP fusion proteins | Live imaging of PAR protein localization and dynamics | Quantitative measurement of domain establishment and boundary stability |
| NMY-2::GFP reporters | Visualization of nonmuscle myosin II dynamics | Assessment of cortical flow during establishment and anterior retention during maintenance |
| Temperature-sensitive alleles | Conditional protein inactivation at restrictive temperature | Staged functional analysis during specific developmental windows |
| Mathematical modeling frameworks | Computational simulation of PAR protein dynamics | Quantitative prediction of how specific mutations affect establishment vs maintenance |
| Cortical intensity quantification tools | Image analysis software for fluorescence measurements | Objective measurement of polarity parameters across multiple embryos |
| Tissue-specific promoters | Cell type-specific manipulation of PAR protein expression | Determination of tissue-specific requirements in gastrulation contexts |
Distinguishing between establishment and maintenance defects in PAR protein mutants requires integrated experimental approaches combining temporal control, quantitative live imaging, and computational modeling. In the context of C. elegans gastrulation, this distinction reveals that PAR proteins function not only in initial axis specification but also in maintaining cellular asymmetries during morphogenetic movements. The experimental frameworks outlined in this guide provide robust methodologies for phenotype classification, enabling researchers to precisely dissect molecular mechanisms governing cell polarity establishment and maintenance. As PAR proteins continue to emerge as important regulators in development and disease, these analytical approaches will prove invaluable for connecting molecular function to phenotypic outcomes.
In the study of C. elegans gastrulation, PAR proteins establish the foundational anterior-posterior polarity of the embryo, a process essential for the subsequent polarization of individual cells during morphogenetic events [23] [32]. A core challenge in this field, as in many genetic studies, is functional redundancy, where the inactivation of a single gene fails to produce a phenotype due to compensatory mechanisms provided by other genes. This phenomenon represents a significant impediment to determining gene function through conventional genetic approaches [33]. For genes with essential roles in early processes like gastrulation, where loss-of-function would be larval lethal, this problem is particularly acute, as it prevents the easy generation and study of mutant strains. This guide outlines strategies to overcome these obstacles, with a specific focus on methodologies applicable within the context of PAR protein research and early embryonic development.
The Challenge of Genetic Redundancy
Genetic redundancy ensures robust regulatory control and protection against mutational assault but obscures gene function for researchers. Estimates suggest that a large proportion of genes may show no obvious phenotype when individually disrupted—up to 85% of genes in a C. elegans RNAi screen failed to produce detectable phenotypes [33]. This "lack of phenotype" can stem from limitations in detection methods or, more commonly, from functional overlap between structurally related proteins or within interconnected pathways [33].
For essential genes required for larval viability or critical processes like gastrulation, this redundancy means that single mutations may not reveal a gene's true function, complicating the analysis of their roles in fundamental biological processes such as cell polarization and ingression.
Key Experimental Strategies
Synthetic Lethal Screening
Synthetic lethal interactions occur when mutations in two genes, each viable alone, cause lethality or a severe defect when combined. This approach is powerful for identifying genes that function in parallel pathways or processes.
Protocol: Targeted Synthetic Lethal Screen [33]
- Strain Engineering: Create a starting strain homozygous for a strong loss-of-function mutation in your gene of interest (e.g.,
lin-35). This strain must also carry an extrachromosomal array (kuEx119) containing:- A wild-type copy of your gene of interest to ensure viability.
- A ubiquitously expressed GFP reporter to visually identify animals carrying the array.
- Mutagenesis: Treat the engineered strain (e.g., MH1461) with a mutagen (e.g., EMS).
- F2 Clonal Screen: Screen the F2 progeny of mutagenized animals for desired mutations. In the F3 generation, look for clear phenotypic differences between GFP-positive (array-bearing, wild-type rescue) and GFP-negative (array-lacking, mutant) progeny.
- Identification and Validation: Isolate mutant strains showing synthetic phenotypes. Confirm the synthetic interaction by using RNAi to knock down the gene of interest in the array-bearing mutant background, which should recapitulate the synthetic lethal phenotype.
Table 1: Key Components for a Targeted Synthetic Lethal Screen
| Component | Example/Description | Function in the Screen |
|---|---|---|
| Mutant Strain | lin-35(n745) |
Provides the homozygous LOF background for the gene under study. |
| Extrachromosomal Array | kuEx119 [lin-35(+), GFP+] |
Provides rescuing function for viability and a visual marker for selection. |
| Mutagen | EMS | Introduces random mutations across the genome to disrupt redundant pathways. |
| Visual Marker | Ubiquitous GFP | Enables rapid sorting of array-bearing (GFP+) and non-bearing (GFP-) animals. |
| Validation Tool | RNAi feeding clones | Confirms the synthetic interaction by phenocopying the mutant allele. |
High-Throughput RNAi Screening
This method allows for the systematic and quantitative identification of synthetic genetic interactions on a genome-wide scale.
Protocol: Quantitative High-Throughput RNAi Synthesis [34]
- Strain Selection: Use a wild-type strain (e.g., N2) and a mutant strain with a weak LOF allele (e.g.,
efl-1(se1)) of your gene of interest. - RNAi Treatment in Liquid Culture: Grow both strains in 96-well plates, each well containing bacteria expressing dsRNA targeting a specific gene from an RNAi feeding library (e.g., targeting all genes of chromosome III).
- Embryo Isolation and Purification:
- After 96 hours, transfer the contents of each well to a 96-well nylon filter plate. Centrifuge briefly (30s at 1000 rpm). Larvae and medium pass through the filter; adults and embryos are retained.
- Resuspend retained worms and embryos in M9 buffer with levamisole to paralyze adults without affecting embryos.
- Centrifuge again to remove the buffer. Transfer the contents to a new filter plate. Without centrifugation, embryos will pass through the filter, while paralyzed adults are retained, yielding a purified embryo culture.
- Automated Phenotyping:
- Image each well of the purified embryo plate immediately (Time 0) and after 24 hours of incubation at 20°C.
- Use automated image analysis software to count the number of embryos at both time points. The difference quantifies embryonic lethality.
- Data Analysis: Compare the percentage of embryonic lethality for each RNAi treatment between the wild-type and mutant backgrounds. Statistically significant differences (e.g., using ANOVA) indicate a synthetic genetic interaction.
Table 2: Synthetic Genetic Interactions of efl-1/E2F with Chromosome III Genes [34]
| Sequence Name | Gene Name | Emb. Leth. Mutant | Emb. Leth. Wild-type | Difference | Statistical Significance |
|---|---|---|---|---|---|
| ZK632.6 | cnx-1 |
0.95 ± 0.02 | 0.31 ± 0.07 | 0.64 ± 0.07 | * |
| F54F2.5 | Ztf-1 |
0.83 ± 0.04 | 0.23 ± 0.04 | 0.60 ± 0.06 | * |
| T26A5.8 | - | 0.65 ± 0.08 | 0.18 ± 0.05 | 0.47 ± 0.10 | * |
| T24C4.1 | ucr-2.3 |
0.64 ± 0.08 | 0.18 ± 0.05 | 0.46 ± 0.09 | * |
| C14B1.8 | - | 0.57 ± 0.04 | 0.17 ± 0.03 | 0.40 ± 0.05 | * |
The Scientist's Toolkit: Key Research Reagents
Table 3: Essential Research Reagents for Analyzing Functional Redundancy
| Reagent | Type/Example | Specific Function in Analysis |
|---|---|---|
| Mutant Strains | lin-35(n745), efl-1(se1), PAR gene mutants (e.g., par-3) |
Provide the genetic background to reveal synthetic interactions with redundant pathways. |
| RNAi Feeding Library | Genome-wide or chromosome-specific clones (e.g., for Chr. III) | Enables systematic knockdown of candidate redundant genes in a high-throughput format. |
| Extrachromosomal Arrays | kuEx119 [lin-35(+), GFP+] |
Allows for the maintenance of lethal mutations in a stock and facilitates screening via co-inherited markers. |
| Molecular Cloning Tools | Gateway vectors, CRISPR-Cas9 systems | For engineering rescue constructs, tagging genes, and creating precise mutations. |
| Automated Imaging Systems | Olympus microscope with automated stage | Enables high-throughput, quantitative phenotyping of embryonic lethality in multi-well plates. |
Integration with PAR Protein Gastrulation Research
The strategies outlined above are directly applicable to dissecting the genetic network surrounding PAR proteins during gastrulation. While PAR proteins themselves are fundamental, their full functional landscape is likely shaped by redundant pathways. For instance, a synthetic lethal screen with a hypomorphic par-3 allele could identify genes that function in parallel to control apical constriction, the driving force behind cell ingression during gastrulation [32]. Similarly, a high-throughput RNAi screen could quantify genetic interactions between core polarity genes and the entire genome, revealing a quantitative genetic network that ensures the robustness of this critical morphogenetic event.
Visualizing Strategic Workflows
Synthetic Lethal Screening Strategy
High-Throughput RNAi Screening Workflow
Overcoming functional redundancy is essential for a complete understanding of genetic networks controlling critical processes like gastrulation. Synthetic lethal screening and quantitative high-throughput RNAi analysis provide powerful, complementary strategies to uncover these hidden genetic relationships. By applying these methods in the context of C. elegans PAR protein research, scientists can systematically identify genes that work in parallel to ensure the robustness of cell polarity and gastrulation, ultimately leading to a more comprehensive model of the genetic control of early embryonic development.
The analysis of protein function in living organisms requires sophisticated tools for conditional and reversible protein disruption. This technical guide details the application of the Auxin-Inducible Degradation (AID) system in C. elegans, with specific focus on its implementation for studying PAR proteins during gastrulation. We provide comprehensive methodologies for implementing both original AID and enhanced AID2 systems, including quantitative comparisons of performance parameters, step-by-step protocols for tissue-specific degradation, and visualization of key signaling pathways. The AID system enables rapid, reversible protein depletion in specific tissues and developmental stages, offering unprecedented temporal and spatial control for functional studies of essential proteins in developing systems.
The PAR Protein Network in Cell Polarization
The PAR (partitioning defective) proteins constitute an evolutionarily conserved system that establishes cellular polarity in diverse biological contexts. First identified in genetic screens for regulators of cytoplasmic partitioning in the early C. elegans embryo, the six core PAR proteins form a fundamental machinery for asymmetric cell division [1]. This network includes PAR-1 and PAR-4 serine-threonine kinases, PAR-3 and PAR-6 scaffold proteins (which form a complex with atypical PKC-3), PAR-5 (a 14-3-3 protein), and PAR-2 (which contains a RING finger domain) [1]. These proteins establish complementary cortical domains through mutual antagonism, creating the anterior-posterior axis in the one-cell embryo [35].
PAR Protein Function in Gastrulation
During C. elegans gastrulation, PAR proteins play critical roles in coordinating cell ingression movements. Research has demonstrated that PAR-3 localizes to apical surfaces of cells prior to blastocoel formation, with this localization determined by cell contacts [23]. Mutations in par-3 cause abnormal separations between embryonic cells, suggesting PAR-3 has a essential role in blastocoel formation during gastrulation [23]. Cells that ingress into the blastocoel undergo apical flattening associated with apical concentration of non-muscle myosin, a process regulated by the PAR network [23]. The ability to precisely disrupt PAR protein function spatiotemporally during gastrulation using the AID system provides a powerful approach to dissect these complex morphogenetic events.
The Auxin-Inducible Degradation (AID) System: Principles and Evolution
Fundamental Mechanism
The AID system harnesses a plant-specific degradation pathway to enable conditional protein depletion in metazoans. The system centers on the F-box protein Transport Inhibitor Response 1 (TIR1), which functions as an auxin-dependent substrate receptor for the Skp1-Cullin-F-box (SCF) ubiquitin ligase complex [36]. When auxin is present, it acts as a molecular glue, promoting interaction between TIR1 and proteins tagged with a short degron sequence (originally from the IAA17 protein of Arabidopsis thaliana). This interaction triggers polyubiquitination and subsequent proteasomal degradation of the target protein [36] [37].
System Evolution: From AID to AID2
Recent advancements have addressed limitations of the original AID system, particularly leaky degradation in the absence of auxin and poor permeability in embryonic stages. The AID2 system incorporates an engineered TIR1(F79G) mutant that exhibits minimal binding to endogenous substrates and high affinity for the synthetic auxin 5-Ph-IAA [38]. This system demonstrates dramatically reduced background degradation and operates effectively with approximately 1,300-fold lower inducer concentrations compared to the original AID system [38].
Table 1: Comparison of AID System Versions
| Parameter | Original AID System | AID2 System |
|---|---|---|
| TIR1 Variant | Wild-type AtTIR1 | Engineered AtTIR1(F79G) |
| Primary Inducer | Indole-3-acetic acid (IAA) | 5-phenyl-indole-3-acetic acid (5-Ph-IAA) |
| Effective Inducer Concentration | High (0.5-4 mM) | Low (0.5-5 μM) |
| Background Degradation | Significant in some cases | Minimal |
| Embryonic Permeability | Limited | Enabled with 5-Ph-IAA-AM analog |
| Time to Depletion | 20-30 minutes | Similar (20-30 minutes) |
| Reversibility | Yes (upon auxin removal) | Yes (upon auxin removal) |
Technical Implementation of AID in C. elegans
Genetic Engineering Requirements
Successful implementation of the AID system requires two genetic components [36] [37]:
TIR1 Expression: A transgenic line expressing the plant TIR1 protein under appropriate regulatory control. Tissue-specific promoters enable spatially restricted degradation capability.
Degron Tagging: The target protein must be tagged with the AID degron sequence using CRISPR/Cas9-based genome editing. Both N-terminal and C-terminal tagging strategies have been successfully employed.
Table 2: Essential Research Reagents for AID Implementation
| Reagent | Function | Examples/Notes |
|---|---|---|
| TIR1 Strains | Expresses the plant F-box protein that recognizes degron-tagged proteins | Available with pan-tissue (eft-3 promoter), tissue-specific (e.g., neuronal, intestinal), or temporally regulated expression |
| Degron Tags | Short sequence that targets fused proteins for degradation | Mini-AID (mAID) or other variants; can be N-terminal or C-terminal |
| Auxin Compounds | Small molecule inducers that trigger degradation | IAA (natural auxin), K-NAA (synthetic), 5-Ph-IAA (AID2), 5-Ph-IAA-AM (embryo-permeable) |
| CRISPR Tools | For introducing degron tags into endogenous loci | Cas9, repair templates with degron sequence, selection markers |
AID Workflow and Mechanism
The following diagram illustrates the complete experimental workflow and molecular mechanism of the AID system:
Tissue-Specific and Temporal Control Strategies
The AID system enables precise spatiotemporal control through strategic selection of promoters driving TIR1 expression [36]:
- Tissue-specific depletion: Using cell-type-specific promoters (e.g., neuronal, intestinal, or germline-specific) to restrict TIR1 expression.
- Temporal control: Employing developmentally regulated or inducible promoters to control when degradation occurs.
- Combined approaches: Layering multiple control elements for sophisticated experimental paradigms.
For embryonic studies, the modified auxin analog 5-Ph-IAA-AM provides enhanced permeability through the eggshell, enabling efficient protein depletion during embryogenesis [38].
Application to PAR Protein Research in Gastrulation
PAR Protein Depletion Strategies
Studying PAR proteins during gastrulation presents unique challenges due to their essential roles in earlier embryonic patterning. The AID system enables bypassing early embryonic lethality through conditional degradation specifically during gastrulation stages. Implementation requires:
- Selection of appropriate TIR1 expression pattern: Use of promoters active during gastrulation but not earlier embryonic stages.
- Timing of auxin application: Administration of auxin at specific developmental timepoints preceding gastrulation.
- Utilization of embryo-permeable auxins: 5-Ph-IAA-AM enables efficient depletion during embryonic stages [38].
PAR Network and Gastrulation Signaling
The following diagram illustrates the PAR protein network and its role in gastrulation, highlighting potential AID targets:
Quantitative Assessment of Depletion Efficacy
Robust evaluation of protein depletion is essential for interpreting phenotypic outcomes. Multiple assessment methods should be employed:
Table 3: Methods for Assessing AID-Mediated Protein Depletion
| Method | Application | Key Parameters |
|---|---|---|
| Live Imaging | Spatial and temporal dynamics of depletion | Fluorescence intensity of tagged proteins, depletion kinetics |
| Western Blotting | Quantitative assessment of total protein levels | Band intensity normalized to controls |
| Immunofluorescence | Tissue-specific depletion efficacy | Signal intensity in specific cell types |
| Phenotypic Scoring | Functional consequences of depletion | Gastrulation defects, cell positioning errors |
| Behavioral Assays | Tissue-specific functional impact | Developmental timing, viability |
Experimental Protocol: AID-Mediated PAR Protein Depletion
Strain Construction and Validation
Step 1: Degron Tagging of Target PAR Genes
- Design CRISPR/Cas9 repair templates to insert degron sequence (mAID preferred for minimal perturbation) at N- or C-terminus of target PAR gene [37].
- Validate tagging by PCR and sequencing, ensuring proper expression and localization of degron-tagged protein.
- Confirm functionality of tagged protein by comparing with wild-type phenotype.
Step 2: Selection of TIR1 Expression Strain
- Choose TIR1 strain with appropriate expression pattern for gastrulation studies.
- For spatial control: Use tissue-specific promoters (e.g., epithelial-specific for gastrulation studies).
- For temporal control: Consider heat-shock or tetracycline-inducible promoters if precise timing is required.
Step 3: Genetic Crosses
- Cross degron-tagged strain with appropriate TIR1-expressing strain.
- Establish homozygous lines carrying both transgenes.
- Include control strains (degron-tagged without TIR1, TIR1 without degron tag) [39].
Auxin Treatment and Phenotypic Analysis
Step 4: Auxin Preparation and Administration
- Prepare NGM plates containing appropriate auxin: IAA (0.5-4 mM) for standard AID, 5-Ph-IAA (0.5-5 μM) for AID2, or 5-Ph-IAA-AM (1-10 μM) for embryonic studies [38] [39].
- Transfer synchronized animals to auxin plates at specific developmental stages preceding gastrulation.
- Include control plates without auxin for comparison.
Step 5: Assessment of Protein Depletion and Phenotypic Analysis
- Monitor depletion kinetics using fluorescence microscopy for tagged proteins.
- For PAR proteins, assess disruption of asymmetric localization patterns.
- Document gastrulation phenotypes: blastocoel formation defects, abnormal cell ingression, mitotic spindle misorientation [23] [11].
- Utilize high-throughput imaging and quantitative analysis for robust phenotypic scoring [35].
Troubleshooting and Optimization
Common Challenges and Solutions
- Incomplete degradation: Optimize auxin concentration; verify TIR1 expression in target tissues; consider alternative degron placement.
- Background degradation in absence of auxin: Switch to AID2 system with TIR1(F79G) and 5-Ph-IAA [38].
- Poor embryonic permeability: Utilize 5-Ph-IAA-AM analog for enhanced embryo penetration [38].
- Pleiotropic effects: Include multiple control strains; verify specificity through rescue experiments.
Quantitative Parameters for System Validation
When implementing AID for PAR protein studies, document these critical parameters:
- Time from auxin addition to detectable protein reduction (typically 20-30 minutes)
- Maximum depletion efficiency (percentage of protein remaining)
- Recovery kinetics after auxin washout
- Tissue-specific variation in depletion efficacy
- Impact on downstream pathway components
The AID system represents a transformative technology for precise protein manipulation in C. elegans, with particular utility for studying essential proteins like PAR network components during gastrulation. The continued refinement of this system, including the development of AID2 with reduced background degradation and enhanced embryonic permeability, provides increasingly sophisticated tools for functional analysis. When properly implemented with appropriate controls and validation, AID-mediated protein depletion enables unprecedented spatial and temporal resolution in probing the roles of PAR proteins and other essential factors in complex developmental processes including gastrulation.
The PAR proteins constitute an evolutionarily conserved machinery fundamental to cell polarization, operating from worms to mammals. Within the context of C. elegans gastrulation, these proteins function as central regulators that seamlessly coordinate apical constriction, cell cycle progression, and cytoskeletal dynamics to ensure the precise cell movements essential for normal development. This review synthesizes current understanding of the PAR protein network, detailing its core molecular circuitry, its interplay with the actomyosin cytoskeleton and cell cycle regulators, and the experimental methodologies that have elucidated its role in polarizing cells during critical morphogenetic events like gastrulation. By framing this discussion within the specific context of C. elegans gastrulation, we aim to provide a mechanistic paradigm for how conserved polarity proteins integrate multiple cellular processes to orchestrate complex developmental outcomes.
The PAR (partitioning defective) proteins were first identified in genetic screens in C. elegans for mutants defective in the asymmetric division of the zygote [1]. Subsequent research has revealed that this group of highly conserved proteins forms a fundamental polarity module that functions across diverse animal species and cellular contexts [40] [1]. In the rapidly developing C. elegans embryo, PAR proteins are indispensable for orchestrating gastrulation, the critical morphogenetic event during which cells destined to form internal tissues move from the embryo's surface into its interior [3]. The gastrulation of the endoderm precursor cells (EPCs) in C. elegans serves as a powerful model for dissecting how PAR proteins coordinate polarity with other cellular processes, as it involves precisely timed apical constriction, a lengthened cell cycle, and dynamic cytoskeletal rearrangements [3]. This review will disentangle the multifaceted roles of PAR proteins, exploring how they integrate signals to polarize cells, regulate their division cycles, and organize the cytoskeleton, with a specific focus on insights gained from the study of C. elegans gastrulation.
Core PAR Protein Network and Molecular Mechanisms
The Core PAR Components and Their Interactions
The core PAR network in C. elegans comprises several key proteins that localize to complementary cortical domains and engage in a complex web of mutual antagonism to establish and stabilize polarity [40] [41]. These can be broadly categorized into anterior PAR proteins (aPARs) and posterior PAR proteins (pPARs).
- Anterior PAR Complex: This group includes the oligomeric scaffold protein PAR-3, the adaptor protein PAR-6, and the atypical protein kinase C (aPKC) PKC-3 [40] [41]. This complex, often in association with the small GTPase CDC-42, becomes enriched at the anterior cortex or, in the case of somatic blastomeres, at apical "caps" on the contact-free membrane surface [40] [11].
- Posterior PAR Proteins: This group includes the serine/threonine kinase PAR-1, the RING domain protein PAR-2, and the tumor suppressor LGL-1 [40]. They localize to the posterior cortex in the zygote, forming a domain complementary to the aPAR complex.
- Symmetrically Localized PAR Proteins: The kinase PAR-4 and the 14-3-3 protein PAR-5 are required for polarity but remain symmetrically distributed, playing crucial regulatory roles in the network [40] [41].
The establishment of polarity is driven by mutual antagonism between the anterior and posterior groups. The aPAR complex, particularly through PKC-3, phosphorylates and excludes posterior components like PAR-1 and PAR-2 from the anterior domain [40]. Conversely, PAR-1 phosphorylates PAR-3, promoting its dissociation from the posterior cortex [40]. PAR-5 facilitates this mutual exclusion by binding to phosphorylated forms of these proteins [40] [1].
Establishing Polarity: Actomyosin Flows and Cortical Dynamics
In the C. elegans zygote, polarity is initiated by a sperm-derived cue that triggers a profound reorganization of the actomyosin cortex [40] [41]. The sperm centrosome locally inhibits RhoA-dependent actomyosin contractility, creating a gradient of cortical tension [40]. This leads to anterior-directed cortical flows, which sweep the aPAR complexes (PAR-3/PAR-6/PKC-3) toward the anterior pole [40] [41] [42]. As the aPARs clear from the posterior, the pPARs (PAR-1, PAR-2, LGL-1) are able to associate with the posterior cortex [41]. This process demonstrates the intimate and reciprocal relationship between the PAR proteins and the cytoskeleton: actomyosin flows establish PAR asymmetry, and the PAR proteins, once localized, subsequently regulate actomyosin dynamics to maintain this asymmetry [41] [42].
- Diagram Title: PAR Protein Polarization and Function in C. elegans
PAR Proteins in Gastrulation: Coordinating Polarity, Cytoskeleton, and Cell Cycle
During C. elegans gastrulation, the roles of PAR proteins extend beyond simple asymmetry establishment to the coordination of multiple cellular processes required for the inward movement of the Endoderm Precursor Cells (EPCs).
Regulation of Apical Constriction and Cytoskeletal Remodeling
The ingression of EPCs is driven by apical constriction, a process powered by the actomyosin cytoskeleton [3]. Non-muscle myosin II (NMY-2) and its phosphorylated regulatory light chain accumulate at the apical surfaces of the ingressing EPCs, causing local contraction of cortical microfilaments [3]. PAR proteins are central regulators of this process. The aPKC PKC-3, as part of the aPAR complex, is a key upstream activator of myosin. Furthermore, the small GTPase CDC-42, which associates with the aPAR complex, acts through the kinase MRCK-1 to activate myosin II, creating gradients of contractility [40]. Inhibition of either microfilaments or myosin activity blocks EPC ingression, underscoring the critical nature of this PAR-cytoskeleton link [3].
Integration with Cell Cycle Control
A distinctive feature of the endoderm lineage is a characteristically longer cell cycle, which allows the EPCs to complete their ingression before dividing [3]. This cell cycle expansion is a pre-programmed event essential for efficient gastrulation. In mutants like gad-1, which fail to expand the E cell cycle, the EPCs divide prematurely and remain on the embryo surface [3]. Significantly, this defect can be rescued by artificially extending the cell cycle, demonstrating that the PAR-dependent polarization machinery and cell cycle control are coordinated to ensure proper morphogenesis [3]. Similar coordination is observed in other systems, suggesting a conserved mechanism whereby cell cycle expansion permits the cytoskeletal machinery to execute cell shape changes and movements without interruption [3].
PAR Proteins and Spindle Orientation in Early Embryos
Recent research has highlighted the role of PAR proteins in orienting the mitotic spindle in symmetrically dividing cells, a process crucial for proper cell positioning in the early embryo. In somatic blastomeres, PAR-3 and aPKC autonomously polarize to form apical caps at the center of the contact-free membrane, independent of cell contacts, actomyosin flows, or microtubules [11]. These apical PAR caps are dynamic structures that influence spindle orientation, thereby contributing to the patterned tissue growth and cell fate specification that underpin subsequent developmental events, including gastrulation [11].
Table 1: PAR Protein Functions in C. elegans Gastrulation and Early Development
| Cellular Process | PAR Protein Involvement | Key Effectors | Functional Outcome |
|---|---|---|---|
| Apical Constriction | aPAR complex (PKC-3, CDC-42) activates myosin II [3] [40]. | NMY-2 (myosin II), F-actin, MRCK-1 [3] [40]. | Constriction of apical surface, driving cell ingression [3]. |
| Cell Cycle Control | Coordination with cell fate specification to lengthen EPC cycle [3]. | GAD-1 (WD repeat protein) [3]. | Allows completion of ingression before cell division [3]. |
| Spindle Orientation | Apical PAR-3/aPKC caps interact with microtubule regulators [11]. | Microtubules, centrosomes, force-generating complexes [11]. | Proper cell positioning and asymmetric division [11]. |
| Cortical Flow | aPARs are transported by flows; PAR-2 inhibits myosin recruitment [40] [41] [42]. | Actomyosin network, RhoA [40] [41]. | Establishment and maintenance of anterior-posterior polarity [40]. |
Experimental Approaches and Key Methodologies
The dissection of PAR protein function has relied on a suite of classical and modern experimental techniques in C. elegans.
Genetic Screens and Mutant Analysis
The original par genes were discovered in pioneering maternal-effect lethal screens designed to find mutants defective in the partitioning of cytoplasmic components [1]. The analysis of these mutants revealed their fundamental role in asymmetric spindle positioning and the unequal segregation of cell fate determinants [41] [1]. This genetic foundation continues to be built upon with reverse genetics approaches like RNA interference (RNAi) to probe the function of newly identified polarity components.
Live-Cell Imaging and Biophysical Analysis
The dynamic nature of PAR proteins has been elucidated through live imaging of fluorescently tagged proteins. Techniques such as Fluorescence Recovery After Photobleaching (FRAP) have demonstrated that PAR proteins are not statically anchored but undergo rapid exchange between the cortex and cytoplasm [40] [41]. Single-molecule imaging has further refined our understanding of their diffusion and transport [40]. These approaches have been critical for observing cortical flows and the real-time dynamics of polarity establishment.
Spatiotemporally Controlled Protein Degradation
A powerful modern method for probing PAR protein function involves the use of auxin-inducible degron systems. This allows for the rapid and specific depletion of PAR proteins at precise developmental timepoints and in specific blastomeres [11]. For example, this technique has been used to demonstrate that PAR-3 and aPKC can form apical caps and regulate spindle orientation even in isolated single blastomeres lacking cell contacts, revealing an intrinsic capacity for symmetry breaking [11].
Blastomere Isolation and Manipulation
Laser ablation and blastomere recombination experiments have been instrumental in distinguishing between cell-autonomous and non-autonomous polarity mechanisms. For instance, by separating, rotating, and rejoining EPCs, researchers showed that neighboring cells (MS and P4) migrate over the EPCs in response to cues from the EPCs themselves, rather than simply chemotaxing toward one another [3].
Table 2: Key Experimental Reagents and Methodologies in PAR Research
| Reagent/Method | Function/Description | Key Application Example |
|---|---|---|
| RNAi (RNA interference) | Gene silencing via introduction of double-stranded RNA [41]. | Knockdown of pkc-3 to confirm its par phenotype [41]. |
| FRAP (Fluorescence Recovery After Photobleaching) | Measures protein dynamics and turnover in living cells [40] [41]. | Demonstrated rapid exchange of cortical PAR-6 and PAR-2 with cytoplasm [41]. |
| Auxin-Inducible Degron | Targeted protein degradation triggered by auxin application [11]. | Spatiotemporal control of PAR-3/aPKC levels to study apical cap formation [11]. |
| GFP-tagged PAR Proteins | Enables live visualization of protein localization and dynamics. | Time-lapse imaging of PAR-6 clearing from posterior cortex during polarization [41]. |
| Laser Ablation | Precise destruction of single cells or structures. | Removal of P4 cell to test its necessity for MS cell migration during gastrulation [3]. |
The Scientist's Toolkit: Research Reagent Solutions
For researchers aiming to investigate PAR protein networks, a core set of reagents and tools is essential. The following table details key solutions for genetic, cell biological, and biochemical experiments in C. elegans.
Table 3: Essential Research Reagents for PAR Protein Studies in C. elegans
| Reagent Category | Specific Examples | Function/Utility |
|---|---|---|
| Genetic Tools | par mutant alleles (e.g., par-3(it71), par-1(b274)) [1]; RNAi feeding clones. | Foundational for loss-of-function studies and genetic interaction analyses. |
| Transgenic Lines | Strains expressing GFP::PAR-3, mCherry::PAR-2, PAR-6::wrmScarlet [41] [11]. | Critical for live imaging of protein localization, dynamics, and cortical flows. |
| Inducible Systems | Auxin-inducible degron tags (e.g., AID::mNeonGreen::PAR-3) [11]. | Enables acute, spatiotemporally controlled protein depletion to study function. |
| Cytoskeletal Probes | Fluorescently tagged Lifeact (F-actin), GFP::NMY-2 (myosin II) [3] [41]. | Visualizes cytoskeletal organization and contractility downstream of PAR proteins. |
| Biochemical Reagents | Inhibitors of myosin (e.g., Blebbistatin), actin (Latrunculin A), or microtubules (Nocodazole) [3] [11]. | Used to dissect the mechanistic contributions of specific cytoskeletal elements. |
The PAR proteins exemplify a conserved, versatile molecular circuit that integrates polarizing cues with fundamental cellular processes to drive morphogenesis. In C. elegans gastrulation, this network links contact-induced cell polarity to the cytoskeletal machinery of apical constriction and the temporal control of the cell cycle, ensuring the precise internalization of the EPCs. While the core circuitry of mutual antagonism between aPARs and pPARs is well-established, future research holds the promise of revealing deeper layers of regulation. Key questions remain: How is the PAR network biochemically wired to the cell cycle clock? What are the full complement of effectors through which PAR proteins orient the mitotic spindle in different developmental contexts? The continued development of sophisticated tools, such as subcellularly targeted protein degradation and high-resolution imaging of protein dynamics, will be crucial for answering these questions. Understanding the intricate coordination managed by the PAR proteins in a model system like C. elegans not only illuminates a fundamental biological principle but also provides insights into the broader dysregulation of polarity that underpins human diseases, including cancer.
Conservation and Context: Validating PAR Circuitry Across Biological Systems
In C. elegans embryogenesis, the partitioning-defective (PAR) proteins constitute a fundamental mechanism for establishing cellular polarity. Among these, PAR-2 plays a critical role during gastrulation, the process by which germ layers become positioned in the embryo. PAR-2, which contains a RING finger domain and may function in the ubiquitination pathway, localizes to the posterior cortex in the one-cell embryo and participates in the mutual exclusion between anterior and posterior PAR complexes [1] [43]. During gastrulation, this polarization is essential for the apical constriction and ingression of endodermal precursor cells, which internalize to form the gut [14]. A key question in evolutionary and developmental biology is whether PAR-2's function is unique to nematodes or if functionally analogous activities exist in other organisms. This guide explores the conservation of PAR-2 and identifies proteins that execute analogous polarization functions across species, providing a technical resource for researchers investigating cell polarity in development and disease.
PAR-2 Function and Conservation Status
Molecular Identity and Role of PAR-2 in C. elegans
The par-2 gene was originally identified in a genetic screen for regulators of cytoplasmic partitioning in the early C. elegans embryo [1]. PAR-2 protein is characterized by a RING finger domain, suggesting a potential role in ubiquitin-mediated protein regulation [1] [43]. During the one-cell stage in C. elegans, PAR-2 becomes enriched in the posterior cortex, where it helps maintain the boundary between anterior and posterior PAR domains through mutual exclusion with the anterior PAR complex (PAR-3/PAR-6/PKC-3) [1]. This polarization is crucial for asymmetric cell division and the proper positioning of developmental determinants.
During gastrulation, the PAR network regulates the apical-basal polarization of cells, which is necessary for the formation of the blastocoel space and the ingression of endodermal precursor cells [14]. PAR-2's role in this process is indirect, as it helps establish the overall cellular polarity that enables subsequent morphogenetic events, including the apical accumulation of non-muscle myosin II (NMY-2) that drives apical constriction [14].
Conservation of PAR Proteins Across Species
Among the core PAR proteins, PAR-2 shows the most limited evolutionary conservation. Research indicates that PAR-2 is a nematode-specific protein with no direct orthologs identified in Drosophila or mammals [43]. This contrasts with other PAR proteins (PAR-1, PAR-3, PAR-4, PAR-5, and PAR-6), which are highly conserved across diverse animal species, including Drosophila and mammals [1] [43].
Table 1: Conservation of PAR Proteins Across Species
| Protein | C. elegans | Drosophila | Mammals | Primary Function |
|---|---|---|---|---|
| PAR-1 | Serine/Threonine kinase | Par-1 | MARK kinases | Kinase regulating microtubule dynamics |
| PAR-2 | RING finger protein | Not conserved | Not conserved | Posterior polarity establishment |
| PAR-3 | PDZ-domain scaffold | Bazooka | mPar3/ASIP | Scaffold for anterior complex |
| PAR-4 | Serine/Threonine kinase | Par-4 | LKB1 | Kinase regulating cell polarity |
| PAR-5 | 14-3-3 protein | Par-5 | 14-3-3 proteins | Phospho-serine binding protein |
| PAR-6 | PDZ-domain scaffold | Par-6 | mPar6 | Scaffold for anterior complex |
| PKC-3 | Atypical PKC | aPKC | aPKC | Kinase in anterior complex |
Despite the lack of direct PAR-2 orthologs, the overall PAR-mediated polarization mechanism is strongly conserved. The functional activities performed by PAR-2 in C. elegans—particularly its role in establishing reciprocal cortical domains—are executed by other molecular players in different biological contexts and organisms.
Functional Analogs of PAR-2 in Other Organisms
PAR-2-like Activities in Drosophila
In Drosophila, the functions analogous to C. elegans PAR-2 are carried out through modifications to the core PAR complex. While Drosophila lacks a direct PAR-2 ortholog, the establishment of opposing cortical domains is achieved through:
Phosphorylation-mediated regulation: PAR-1 kinase phosphorylates the anterior protein PAR-3 (Bazooka in Drosophila), creating binding sites for 14-3-3 proteins (PAR-5) that exclude PAR-3 from the posterior cortex [1]. This phosphorylation-dependent exclusion mechanism functionally replaces PAR-2's role in C. elegans.
aPKC-mediated inhibition: The anterior complex component aPKC phosphorylates and excludes PAR-1 from the anterior domain, reinforcing the boundary between opposing PAR domains [1].
This reciprocal phosphorylation and exclusion system in Drosophila achieves the same functional outcome as the PAR-2-dependent system in C. elegans, demonstrating evolutionary conservation of the mechanism despite molecular differences.
PAR-2-like Activities in Mammalian Systems
Mammalian cells utilize several conserved mechanisms to establish polarity analogous to PAR-2 function:
PAR-1/MARK kinases: Mammalian PAR-1 homologs (MARK kinases) phosphorylate mammalian PAR-3, leading to 14-3-3 binding and cortical exclusion, mirroring the Drosophila mechanism [43].
The LKB1-PAR-4 pathway: In mammalian neurons, LKB1 (the mammalian PAR-4 ortholog) phosphorylates and activates PAR-1 homologs, establishing neuronal polarity through mechanisms that parallel PAR-2 functions in C. elegans [43].
aPKC regulation: Mammalian aPKC phosphorylates and excludes PAR-1 from apical domains in epithelial cells, maintaining the boundary between apical and basolateral membrane domains [44].
Table 2: Functional Analogs of PAR-2 Across Species
| Organism | Functional Analog | Molecular Mechanism | Biological Context |
|---|---|---|---|
| C. elegans | PAR-2 | RING finger domain protein; excludes anterior PAR complex from posterior cortex | Early embryo polarization; gastrulation |
| Drosophila | PAR-1 kinase | Phosphorylates PAR-3/Bazooka, leading to 14-3-3 mediated exclusion | Neuroblast asymmetric division; oocyte polarization |
| Mammals | MARK kinases (PAR-1) | Phosphorylate mPAR-3, creating 14-3-3 binding sites | Epithelial cell polarity; neuronal polarization |
| Mammals | LKB1 (PAR-4) | Phosphorylates and activates MARK kinases | Epithelial morphogenesis; neuronal polarity |
Experimental Approaches for Identifying PAR-2-like Activities
Genetic Screening and Epistasis Analysis
Traditional genetic approaches remain powerful for identifying PAR-2-like activities:
Protocol: Enhancement/Suppression Screening for Polarity Mutants
- Begin with a hypomorphic allele of a core polarity gene (e.g., par-3 or par-6) with mild polarity defects.
- Perform mutagenesis using EMS (ethyl methanesulfonate) or insertional mutagens.
- Screen for mutants that enhance or suppress the polarity phenotype.
- Map and identify the mutated genes through whole-genome sequencing or complementation testing.
- Test genetic interactions by constructing double mutants with known par genes.
This approach successfully identified the original par mutants in C. elegans and can reveal components of the same functional pathway in other organisms [1].
Live Imaging of Protein Localization
Visualizing the dynamics of polarity proteins is essential for identifying PAR-2-like activities:
Protocol: Time-Lapse Imaging of Cortical Polarity
- Generate transgenic lines expressing fluorescently tagged PAR proteins (e.g., GFP-PAR-3, mCherry-PAR-1).
- For embryonic studies, dissect embryos and mount them in appropriate chambers for live imaging.
- Acquire time-lapse images using spinning-disk or confocal microscopy with high temporal resolution (30-60 second intervals).
- Quantify fluorescence intensity at the cell cortex using image analysis software (e.g., ImageJ/FIJI).
- Perturb candidate PAR-2-like proteins via RNAi or CRISPR/Cas9 and assess changes in PAR domain establishment and maintenance.
This method revealed that PAR-3 and PAR-6 remain apical during intestinal epithelial cell division in C. elegans while microtubules are transiently removed, demonstrating the conservation of apical-basal polarization mechanisms [44].
Biochemical Analysis of Protein Complexes
Identifying physical interactions between polarity proteins can reveal functional analogs:
Protocol: Co-Immunoprecipitation of PAR Complexes
- Prepare cell lysates from polarized epithelial cells or embryonic tissues.
- Incubate lysates with antibodies against known PAR proteins (e.g., PAR-3, PAR-6, aPKC).
- Capture immune complexes using protein A/G beads.
- Wash beads extensively and elute bound proteins.
- Analyze co-precipitating proteins by Western blotting or mass spectrometry.
- Test phosphorylation-dependent interactions using phosphatase treatment or phosphomimetic mutants.
This approach identified the conserved PAR-3/PAR-6/aPKC complex and its regulation by CDC42 in diverse cell types [44].
Research Reagent Solutions
Table 3: Essential Research Reagents for Studying PAR-2-like Activities
| Reagent Category | Specific Examples | Function/Application |
|---|---|---|
| Antibodies | Anti-PAR-3, Anti-PAR-6, Anti-aPKC, Anti-PAR-1/MARK | Protein localization by immunofluorescence; Western blot analysis |
| Genetic Tools | par-2 RNAi constructs, PAR-2 CRISPR/Cas9 knockout vectors, PAR-2 transgenic rescue constructs | Functional perturbation and analysis of PAR-2 requirements |
| Live Imaging Reagents | GFP-PAR-2, mCherry-PAR-1, H2B-GFP (chromatin marker) | Real-time visualization of protein dynamics during polarization |
| Biochemical Reagents | Phospho-specific PAR-3 antibodies, 14-3-3 binding inhibitors, aPKC inhibitors | Analysis of phosphorylation-dependent regulatory mechanisms |
| Model Organisms | C. elegans N2 (wild-type), par-2 mutant strains, Drosophila PAR-1 mutants, Mammalian epithelial cell lines | Comparative studies of polarity mechanisms across species |
Signaling Pathway Diagrams
While PAR-2 itself is not conserved beyond nematodes, the functional activities it performs in establishing cellular polarity are carried out by alternative mechanisms in other organisms, primarily through the coordinated actions of PAR-1 kinase, PAR-4/LKB1, and regulation of the anterior PAR complex. Identifying these functional analogs requires a multifaceted approach combining genetic screening, live imaging, and biochemical analysis.
Future research should focus on:
- Systematic comparison of phosphorylation networks in different polarity systems
- Identification of novel RING domain proteins that may function similarly to PAR-2 in mammalian cells
- Exploration of potential functional analogs in three-dimensional organoid systems
- Investigation of how PAR-2-like activities contribute to disease states, particularly in cancer and neurological disorders where cell polarity is disrupted
Understanding these conserved mechanisms provides fundamental insights into how cells establish asymmetry—a process critical for development, tissue homeostasis, and disease pathogenesis.
The PAR (partitioning defective) proteins, discovered for their role in asymmetric cell division in the C. elegans zygote, are fundamental regulators of cell polarity across diverse animal species. While their embryonic functions are well-documented, their postembryonic roles remain less explored. This review focuses on the essential functions of two core PAR complex components, PAR-6 and PKC-3, during larval development. Evidence from targeted protein degradation studies reveals that PAR-6 and PKC-3, but not PAR-3, are indispensable for postembryonic development, functioning within the epidermal epithelium to coordinate animal growth, molting cycles, and stem cell division patterns. Furthermore, we explore a novel role for PAR-6 in organizing non-centrosomal microtubules through the recruitment of the microtubule organizer NOCA-1/Ninein. These findings significantly expand our understanding of PAR protein functionality beyond embryonic patterning and establish their critical importance in larval development and tissue morphogenesis.
The PAR proteins were first identified in genetic screens for regulators of cytoplasmic partitioning in the early embryo of C. elegans [1]. Six PAR genes were discovered, encoding proteins that establish an anterior-posterior polarity axis in the one-cell zygote, enabling asymmetric cell divisions that segregate developmental determinants to appropriate daughter cells [1]. This foundational polarization event is essential for normal embryogenesis, positioning the PAR proteins as master regulators of cellular asymmetry.
The molecular identities of the PAR proteins revealed their potential for complex signaling interactions. PAR-1 and PAR-4 encode serine-threonine kinases, PAR-5 is a 14-3-3 family protein, PAR-2 contains a RING finger domain, while PAR-3 and PAR-6 are scaffold proteins containing PDZ domains [1]. PAR-6, atypical Protein Kinase C (aPKC, known as PKC-3 in C. elegans), and PAR-3 form a highly conserved complex that localizes to the anterior cortex of the one-cell embryo, opposing the posterior localization of PAR-1 and PAR-2 [1]. This asymmetric distribution creates molecularly distinct domains that ultimately dictate cell fates.
Beyond the zygote, PAR proteins polarize various cell types during embryogenesis, including migrating cells and epithelial cells [1]. In epithelial cells, which polarize along an apicobasal axis, the PAR-6/PKC-3/PAR-3 complex becomes a key determinant of apical identity and is required for the formation and maintenance of apical junctions [29] [24]. Surprisingly, despite extensive study of their embryonic functions, roles for PAR proteins during larval development have remained largely unexplored until recently. The development of tissue-specific protein degradation tools has now enabled researchers to investigate these essential genes in postembryonic contexts, revealing critical requirements in the larval epidermis that are the focus of this review.
The Core PAR Complex: Molecular Architecture and Mechanisms
The PAR-6/PKC-3/PAR-3 complex forms the central machinery for establishing cellular asymmetry. Each component possesses distinct protein-interaction domains that facilitate complex assembly, regulation, and connection to downstream effectors.
Domain Organization and Key Interactions
- PAR-6: This scaffold protein contains a PB1 domain that mediates interactions with PKC-3, a CRIB domain that binds the small GTPase CDC-42, and a PDZ domain that can recruit additional signaling proteins [45].
- PKC-3 (aPKC): An atypical protein kinase C that binds PAR-6 via its own PB1 domain and phosphorylates downstream targets to establish apical identity, including PAR-3 itself, which excludes it from the apical domain in mature epithelia [29].
- PAR-3: This multi-PDZ domain scaffold protein can oligomerize and bind both PAR-6 and lipid membranes, facilitating the clustering of the PAR complex at specific cortical sites [29].
The dynamic interactions between these components, regulated by phosphorylation and GTPase activity, allow the complex to establish and maintain polarized membrane domains. In particular, CDC-42 binding to PAR-6 promotes a conformational change that activates PKC-3 kinase activity, enabling phosphorylation of downstream substrates that define apical character [29] [45].
Figure 1: Molecular Architecture and Functional Relationships of the Core PAR Complex. The diagram illustrates the protein-interaction domains mediating complex assembly and key regulatory interactions that establish cell polarity and organize microtubules.
Context-Dependent Requirements in Epithelia
While the core complex is conserved, genetic analyses reveal tissue-specific requirements for its components. In embryonic epithelia, PAR-6 is required for apical junction formation in all epithelial tissues, but the extent of polarization defects varies [29]. Similarly, PAR-3 is essential for junction formation in intestinal and pharyngeal epithelia but is dispensable in the embryonic epidermis [29] [24]. This context dependence highlights the adaptability of the PAR network and suggests the existence of tissue-specific effectors and redundant mechanisms.
Essential Larval Functions of PAR-6 and PKC-3 in the Epidermis
The development of the auxin-inducible degradation (AID) system for tissue-specific protein depletion has enabled the functional dissection of essential genes like par-6 and pkc-3 during larval stages. This approach involves tagging endogenous proteins with an AID degron, allowing for their rapid degradation upon expression of the plant TIR1 ubiquitin ligase in specific tissues [29].
Experimental Approach: Targeted Protein Degradation
Methodology for Inducible Protein Depletion:
- Strain Generation: CRISPR/Cas9 was used to insert sequences encoding the AID-degron and GFP into the endogenous par-6, pkc-3, and par-3 loci, ensuring all protein isoforms were tagged [29].
- Tissue-Specific Depletion: The TIR1 ubiquitin ligase was expressed under tissue-specific promoters (e.g., epidermal-specific wrt-2 promoter, intestinal-specific elt-2 promoter) [29].
- Induction of Degradation: Animals were exposed to auxin, triggering the degradation of AID-tagged proteins in TIR1-expressing tissues.
- Phenotypic Analysis: Depleted animals were assessed for developmental progression, molting, cell division patterns, and cellular organization.
PAR-6 and PKC-3 Are Indispensable for Postembryonic Development
Ubiquitous depletion of PAR-6 and PKC-3 resulted in complete larval arrest, demonstrating their essentiality for postembryonic development [29]. In contrast, PAR-3 depletion had no overt effect on larval viability, indicating divergent requirements within the PAR complex [29]. Tissue-specific depletion revealed that the epidermis is the critical tissue requiring PAR-6 and PKC-3 function.
Table 1: Phenotypic Consequences of Epidermal PAR-6/PKC-3 Depletion in C. elegans Larvae
| Phenotypic Category | Specific Defects | Functional Implication |
|---|---|---|
| Growth & Development | Larval growth arrest; Failure to reach adulthood | Essential role in supporting developmental progression |
| Molting Cycle | Failure to shed old cuticle; Defects in cuticle synthesis | Disruption of extracellular matrix remodeling and synthesis |
| Seam Cell Divisions | Altered division patterns; Loss of asymmetric divisions | Defective stem cell lineage patterning and tissue expansion |
| Cell Junctions | Defects in apical junction maintenance; Altered apical domain identity | Compromised epithelial barrier function and polarity |
| Cortical Organization | Failure to exclude LGL-1 from apical domain | Disrupted apical-basal polarity establishment |
Epidermal depletion of PAR-6 or PKC-3 recapitulated the larval arrest phenotype, accompanied by specific failures in molting and seam cell division patterning [29]. The seam cells, which function as epidermal stem cells, normally undergo asymmetric divisions to generate both new seam cells and differentiated hypodermal cells. Upon PAR-6 or PKC-3 depletion, this pattern is severely disrupted, compromising epidermal expansion and tissue integrity [29]. Furthermore, depleted animals failed to exclude the basal determinant LGL-1 from the apical domain, confirming their role in establishing and maintaining cortical asymmetry [29].
A Novel Mechanism: PAR-6 in Organizing Non-Centrosomal Microtubules
Beyond its established role in polarity, a groundbreaking discovery reveals that PAR-6 organizes non-centrosomal microtubule arrays in the epidermis, uncovering a previously unrecognized function connecting cortical polarity with cytoskeletal architecture.
Microtubule Organization Defects in PAR-6-Depleted Epidermis
In polarized epithelial cells, microtubules are typically organized into non-centrosomal arrays that run along the apicobasal axis, providing structural support and serving as tracks for intracellular transport. Epidermal depletion of PAR-6 resulted in severe disorganization of these microtubule arrays, with a notable loss of aligned microtubule bundles [29]. This defect was specific to PAR-6 and PKC-3 depletion, as PAR-3 depletion did not disrupt microtubule organization, mirroring their differential requirements for larval viability [29].
The Microtubule Organizer NOCA-1/Ninein as a PAR-6 Effector
The mechanistic link between PAR-6 and microtubules was identified as NOCA-1, the C. elegans homolog of the conserved microtubule anchor protein Ninein. NOCA-1 localizes to the apical epidermis in a PAR-6-dependent manner, and its loss in noca-1 mutants phenocopies the microtubule defects observed in PAR-6-depleted animals [29]. Furthermore, NOCA-1 physically interacts with PAR-6, suggesting a direct mechanism for microtubule organizer recruitment [29].
Additional evidence supporting this mechanism includes:
- Co-localization: PAR-6 and NOCA-1 show overlapping localization patterns in the epidermal epithelium.
- Dependent localization: NOCA-1 apical localization is lost upon PAR-6 depletion.
- Similar mutant phenotypes: noca-1 mutants and PAR-6-depleted animals exhibit nearly identical microtubule disorganization.
- Physical interaction: Biochemical assays confirm a direct molecular interaction between PAR-6 and NOCA-1.
These findings support a model wherein PAR-6, through the recruitment of NOCA-1/Ninein, establishes a cortical platform for the nucleation and stabilization of non-centrosomal microtubules, thereby coordinating cell polarity with cytoskeletal organization.
Table 2: Key Proteins Linking PAR-6 to Microtubule Organization
| Protein | Identity/Function | Role in Microtubule Organization | Genetic/Physical Interaction with PAR-6 |
|---|---|---|---|
| NOCA-1 | Ninein homolog; Microtubule anchor | Nucleates and stabilizes non-centrosomal microtubules | Direct physical interaction; Required for apical localization |
| GIP-1 | γ-Tubulin Ring Complex component | Promotes microtubule nucleation | Localization lost in PAR-6 depleted epidermis |
| PTRN-1 | Patronin/CAMSAP homolog | Stabilizes microtubule minus ends | Localization disrupted upon PAR-6 depletion |
| PKC-3 | aPKC; Kinase component | Phosphoregulation of complex assembly | Forms core complex with PAR-6; Required for function |
Figure 2: PAR-6-Dependent Pathway for Non-Centrosomal Microtubule Organization. The model illustrates how PAR-6 recruits NOCA-1/Ninein to the apical cortex, which in turn promotes the localization of microtubule nucleating (GIP-1/γ-TuRC) and stabilizing (PTRN-1/Patronin) factors to organize apicobasal microtubule arrays.
The Scientist's Toolkit: Key Research Reagents and Methodologies
Advancing research in PAR protein biology requires specialized reagents and tools. The following table summarizes key resources for investigating PAR-6/PKC-3 function in C. elegans.
Table 3: Essential Research Reagents for Studying PAR-6/PKC-3 Function
| Reagent/Tool | Type | Key Features/Applications | Example Strain/Identifier |
|---|---|---|---|
| AID-System Strains | Genetically modified C. elegans | Enables tissue-specific, auxin-inducible degradation of AID-tagged proteins | par-6(mib30[par-6::aid::egfp-loxp]) I; ieSi57[eft-3p::TIR1::mRuby::unc-54 3'UTR] II [29] |
| Endogenous GFP Tagged PAR-6 | CRISPR-modified allele | Visualizes protein localization and dynamics in live animals | par-6(mib30[par-6::aid::egfp-loxp]) I [29] |
| Endogenous GFP Tagged PKC-3 | CRISPR-modified allele | Enables live imaging of PKC-3 distribution and abundance | pkc-3(mib78[egfp-loxp::aid::pkc-3]) II [29] |
| Epidermal-Specific TIR1 Strains | Tissue-specific degradation driver | Targets protein depletion specifically to epidermal cells | mibIs49[Pwrt-2::TIR-1::tagBFP2-Lox511::tbb-2 3'UTR] IV [29] |
| Microtubule Reporter Strains | Fluorescent protein fusions | Visualizes microtubule organization and dynamics in vivo | maph-1.1(mib12[egfp::maph-1.1]) I [29] |
| Junction Marker Strains | Fluorescent protein fusions | Monitors cell junction integrity and apical domain organization | dlg-1(mib23[dlg-1::mCherry-LoxP]) X [29] |
Critical Methodological Considerations
When employing these reagents, several methodological aspects require attention:
- Degradation Efficiency: Auxin concentration and exposure time must be optimized for complete degradation while minimizing non-specific effects.
- Tissue Specificity: Promoter choice for TIR1 expression is critical; the wrt-2 promoter provides strong epidermal specificity [29].
- Phenotypic Timing: Developmental timing of depletion is crucial, as early larval depletion may cause more severe defects than later depletion.
- Genetic Background: Proper control strains (lacking TIR1 or AID tag) must be included to account for potential auxin effects unrelated to degradation.
Concluding Perspectives and Future Directions
The essential roles of PAR-6 and PKC-3 in larval epidermal development underscore the enduring importance of polarity machinery beyond embryogenesis. The discovery that PAR-6 organizes non-centrosomal microtubules through NOCA-1/Ninein recruitment reveals a novel mechanism linking cortical polarity to cytoskeletal organization, with potential implications for understanding epithelial biology across species.
Several promising research directions emerge from these findings:
- Signaling Integration: How do PAR-6 and PKC-3 integrate diverse signaling inputs to coordinate polarity with tissue growth and differentiation?
- Disease Connections: Given the role of human Par6 in tumor development and metastasis [45], do the newly identified microtubule organizing functions contribute to disease processes?
- Mechanotransduction: Could the PAR-6-microtubule axis participate in sensing and responding to mechanical forces within epithelial tissues?
- Temporal Regulation: How is PAR complex function modulated to accommodate different requirements during embryonic versus larval development?
These questions highlight the dynamic nature of polarity regulation and position the C. elegans larval epidermis as a powerful model for unraveling the complex interplay between cell polarity, cytoskeletal dynamics, and tissue morphogenesis.
The PAR cell polarity proteins are fundamental regulators of asymmetric cell division and tissue morphogenesis. Emerging evidence reveals a conserved effector pathway through which PAR complexes directly orchestrate the organization of non-centrosomal microtubule arrays via the ninein-like protein NOCA-1. This whitepaper synthesizes recent advances elucidating the PAR-6/PKC-3 → NOCA-1 signaling axis in C. elegans, detailing its mechanistic role in gastrulation and other morphogenetic processes. We present comprehensive experimental data, methodological protocols, and visualization tools to empower research into this critical pathway connecting cell polarity with microtubule-mediated cellular architecture.
The partitioning defective (PAR) proteins constitute an ancient, conserved system for establishing cellular asymmetry in diverse biological contexts [1]. Initially identified through genetic screens in C. elegans for regulators of cytoplasmic partitioning, the six core PAR proteins form an integrated network that polarizes cells through mutual exclusion and targeted localization [1]. PAR proteins function as central signaling nodes, translating spatial information into cytoskeletal reorganization and asymmetric cell divisions essential for development.
Within the framework of C. elegans gastrulation, PAR proteins undergo a critical transition from anterior-posterior polarization in the one-cell embryo to apical-basal polarization in multicellular stages [46]. This repolarization establishes the fundamental axis for morphogenetic movements, directing the apical constriction and ingression of endodermal precursors that initiate gastrulation [46] [23]. The PAR network, particularly the anterior PAR complex (PAR-3/PAR-6/PKC-3), becomes enriched at apical surfaces, positioning it to coordinate downstream effectors that execute mechanical aspects of tissue remodeling [46].
Molecular Mechanism: PAR-6/PKC-3 Control of NOCA-1 and Microtubule Organization
Core Pathway Architecture
Recent research has identified a direct molecular pathway through which PAR proteins govern the assembly of non-centrosomal microtubule (MT) arrays. In larval epidermal cells of C. elegans, the PAR-6/PKC-3 complex is essential for recruiting NOCA-1 (the nematode ninein homolog) to cortical sites [29]. NOCA-1 subsequently recruits the γ-tubulin ring complex (γ-TuRC) component GIP-1 and the minus-end stabilizing protein PTRN-1 (CAMSAP/Patronin) [29]. This hierarchical pathway organizes circumferential microtubule arrays that resist mechanical deformation during embryonic elongation and are required for proper morphogenesis.
Figure 1: PAR-6/PKC-3 Control of Non-Centrosomal Microtubule Organization via NOCA-1
Functional Relationships in Different Cellular Contexts
The functional output of the PAR-6 → NOCA-1 pathway varies across tissues and developmental stages, reflecting context-specific requirements for microtubule organization:
Table 1: Tissue-Specific Functions of the PAR-6/NOCA-1 Pathway
| Tissue/Cell Type | Microtubule Array Organization | Biological Function | Genetic Interactions |
|---|---|---|---|
| Larval Epidermis | Circumferential arrays nucleated from hemidesmosomes and adherens junctions | Embryonic elongation, junctional protein transport (E-cadherin, myotactin) | Functions with LET-502/ROCK; parallel to PTRN-1 [47] [29] |
| Germline | Cortical arrays at cell surface | Nuclear positioning, germline organization | Requires γ-tubulin; independent of PTRN-1 [48] |
| Neurons (PVD) | Minus-end-out dendritic arrays | Dendrite polarity, neuronal development | Functions with NOCA-2; parallel to PTRN-1 [49] |
| Embryonic Epidermis | Apical-basal arrays | Gastrulation, apical constriction | Downstream of PAR-3/PAR-6 polarity [46] [23] |
Experimental Evidence: Key Findings and Quantitative Data
Genetic and Cell Biological Analysis
Multiple experimental approaches have established the functional hierarchy of the PAR-6/NOCA-1 pathway. Inducible degradation of PAR-6 or PKC-3 in larval epithelia results in complete dislocalization of NOCA-1 from cortical sites, followed by disrupted γ-tubulin and PTRN-1 localization [29]. Crucially, loss of NOCA-1 phenocopies the microtubule organization defects observed upon PAR-6 depletion, positioning NOCA-1 as the primary effector of PAR-6/PKC-3 in microtubule regulation [29].
Physical interaction between PAR-6 and NOCA-1 provides the molecular basis for this regulatory relationship. Protein-binding assays confirm direct association, suggesting a mechanism for cortical targeting independent of PAR-3 [29]. This PAR-3 independence represents a significant divergence from canonical PAR complex function and highlights the context-specific organization of polarity networks.
Table 2: Quantitative Phenotypic Analysis of Pathway Component Depletion
| Genetic Manipulation | Embryonic Elongation Defects | Microtubule Regrowth (15s post-cold shock) | Gastrulation Defects | Larval Lethality |
|---|---|---|---|---|
| PAR-6 depletion | Severe (2.5-fold arrest) [29] | 92% reduction at junctions [29] | Not quantified | 100% [29] |
| NOCA-1 depletion | Moderate (3-fold arrest) [48] [47] | 87% reduction at junctions [47] | Not quantified | 45% [48] |
| γ-tubulin depletion | Moderate (3-fold arrest) [48] [47] | 95% reduction at junctions [47] | Not quantified | 65% [48] |
| PTRN-1 depletion | Mild (3.5-fold arrest) [48] | 22% reduction at junctions [47] | Not quantified | 15% [48] |
| NOCA-1; PTRN-1 double depletion | Severe (2-fold arrest) [48] | 98% reduction at junctions [47] | Not quantified | 92% [48] |
Integration with Gastrulation Mechanisms
During C. elegans gastrulation, the PAR-6/NOCA-1 pathway functions within endodermal precursor cells to facilitate apical constriction, the primary mechanical driver of cell ingression. PAR-6 localizes to the apical domain of these cells, where it directs NOCA-1-dependent organization of non-centrosomal microtubules [46]. These microtubule arrays contribute to the trafficking of junctional components and potentially stabilize the constricting apical surface against mechanical stress.
The pathway intersects with Wnt/Frizzled signaling, which activates actomyosin contractility through phosphorylation of myosin regulatory light chain [50]. While Wnt signaling directly controls the contractile machinery, the PAR-6/NOCA-1 axis ensures proper cellular architecture through microtubule organization, creating a coordinated system that links cell fate specification with morphogenetic execution.
Methodological Approaches: Experimental Protocols
Protein Depletion and Phenotypic Analysis
Auxin-Inducible Degradation of PAR-6, PKC-3, and NOCA-1:
- Genetic Engineering: Generate strains expressing AID-degron tagged proteins using CRISPR/Cas9. Tag PAR-6 at its C-terminus, PKC-3, and NOCA-1 to preserve functional domains [29].
- Depletion Protocol: Synchronize larvae at L1 stage and transfer to NGM plates containing 4 mM auxin (indole-3-acetic acid). Incubate for 6-24 hours at 20°C depending on desired depletion duration [29].
- Validation: Monitor degradation efficiency by fluorescence microscopy of GFP-tagged proteins. Typically >90% depletion achieved within 4 hours of auxin exposure [29].
Microtubule Regrowth Assay:
- Cold-Induced Depolymerization: Incubate embryos or larvae at 4°C for 30 minutes to depolymerize microtubules [47].
- Regrowth: Shift to room temperature (22°C) and allow microtubule regrowth for precisely 15 seconds [47].
- Fixation and Imaging: Immediately fix samples in -20°C methanol for 10 minutes, then stain with anti-α-tubulin antibodies (1:500 dilution) and anti-γ-tubulin antibodies (1:250 dilution) to visualize nucleation sites [47].
Microtubule Dynamics and Polarity Analysis
EB1/EBP-2 Tracking for Microtubule Growth Directionality:
- Live Imaging: Express EBP-2::GFP in epidermal or neuronal cells using tissue-specific promoters [47].
- Image Acquisition: Capture time-lapse images at 2-second intervals for 5 minutes using TIRF or spinning-disk confocal microscopy [47].
- Trajectory Analysis: Track EBP-2 comet movements using plusTipTracker software (MATLAB) or equivalent. Calculate growth angles relative to cellular axes to determine preferential orientation [47].
Microtubule Polarity Assay in Neurons:
- Marker Expression: Express plus-end (EBP-2::GFP) and minus-end (NOCA-2::mCherry or PTRN-1::GFP) markers in PVD neurons [49].
- Image Acquisition: Acquire high-resolution z-stacks of dendritic and axonal processes using confocal microscopy [49].
- Polarity Quantification: Calculate the percentage of microtubules with minus-end-out orientation in dendrites versus plus-end-out in axons. Wild-type dendrites typically show >80% minus-end-out organization [49].
Figure 2: Experimental Workflow for Analyzing PAR-6/NOCA-1 Pathway Function
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for Investigating PAR/NOCA-1 Pathway
| Reagent/Resource | Type | Function/Application | Source/Reference |
|---|---|---|---|
| PAR-6::AID::GFP | CRISPR-tagged strain | Auxin-inducible degradation of PAR-6 with visual monitoring | [29] |
| NOCA-1::GFP | Endogenously tagged protein | Localization studies of ninein homolog | [48] [47] |
| PTRN-1::GFP | Fluorescent protein fusion | Visualization of microtubule minus-end stabilization | [49] [47] |
| γ-tubulin::GFP | Fluorescent reporter | Identification of microtubule nucleation sites | [48] [47] |
| EBP-2::GFP | Plus-end binding protein | Live imaging of microtubule polymerization dynamics | [47] |
| Auxin (IAA) | Chemical inducer | Triggered degradation of AID-tagged proteins (4mM working concentration) | [29] |
| Anti-α-tubantibody | Immunoreagent | Microtubule visualization in fixed samples | [47] |
| Spastin overexpression construct | Microtubule disruption tool | Inducible severing of microtubules | [47] |
The PAR-6/PKC-3 → NOCA-1 pathway represents a conserved effector mechanism that directly links cell polarity with microtubule cytoskeleton organization. In C. elegans gastrulation and embryonic development, this axis ensures proper cellular architecture, junctional remodeling, and resistance to mechanical stress. The experimental frameworks and reagents detailed herein provide a foundation for further investigation into how polarity information is transduced into cytoskeletal architecture.
Future research should address several outstanding questions: How is PAR-6 activity regulated to control NOCA-1 localization? What additional effector pathways operate in parallel to coordinate actin and microtubule networks? How conserved is this mechanism in vertebrate epithelial polarization and morphogenesis? Answering these questions will further illuminate the fundamental principles through which cells translate polarity signals into structural organization and mechanical function.
The PAR protein network constitutes a deeply conserved engine for cell polarization, operating across metazoans to establish cellular asymmetries essential for development. This whitepaper examines the core biochemical circuitry of the PAR system, with specific focus on its role in C. elegans gastrulation. We explore how a conserved set of scaffolding proteins, adaptors, and enzymes—PAR-1, PAR-2, PAR-3, PAR-4, PAR-5, PAR-6, PKC-3, and CDC-42—can be reconfigured through tuned interactions, post-translational modifications, and geometric sensing to respond to diverse polarizing inputs. The analysis synthesizes recent advances in live-cell imaging, biophysical analyses, and mathematical modeling that reveal how this fundamental polarity module is adapted to specific developmental contexts, with particular emphasis on the mechanistic insights relevant to drug development targeting cell polarization pathways.
The PAR (partitioning defective) proteins were first identified in genetic screens for regulators of cytoplasmic partitioning in the early C. elegans embryo [1]. These proteins form a highly conserved network of scaffolds, adaptors, and enzymes that control cell polarity across diverse developmental and physiological contexts throughout metazoa [40]. A key feature of the PAR network is the asymmetric localization of its components in polarized cells, where they establish and maintain cortical asymmetries in response to various polarizing cues.
In the decades since their initial discovery, research has revealed that PAR proteins constitute a versatile polarity module that has been adapted for numerous cellular functions beyond the initial asymmetric division of the C. elegans zygote. These include apico-basal polarization in epithelial cells, planar cell polarity, asymmetric cell divisions in stem cells, and cell migration [40] [1]. The core PAR circuitry appears to be fundamentally conserved, but its regulation and connectivity with downstream effectors are tuned according to cellular context and functional requirements.
This technical guide examines the molecular machinery of the PAR protein network, with specific focus on how its core components and their interactions are modulated to generate distinct polarity outcomes in different developmental contexts, particularly during C. elegans gastrulation. We integrate biochemical, cell biological, and computational perspectives to provide researchers with a comprehensive understanding of this fundamental polarity system.
Core PAR Protein Components and Their Biochemical Functions
The PAR network comprises several highly conserved proteins that form the core polarity machinery. These proteins can be categorized based on their localization patterns and functional relationships.
Table 1: Core PAR Protein Components and Their Biochemical Functions
| Protein | Molecular Identity | Primary Function | Localization in C. elegans Zygote | Conservation |
|---|---|---|---|---|
| PAR-1 | Serine/threonine kinase | Phosphorylates PAR-3, regulates microtubule dynamics | Posterior cortex | High (MARK kinases) |
| PAR-2 | RING finger domain protein | E3 ubiquitin ligase activity, recruits PAR-1 to membrane | Posterior cortex | Limited (functional analogs may exist) |
| PAR-3 | PDZ domain scaffold protein | Oligomerizes, nucleates anterior complex | Anterior cortex | High (Bazooka in Drosophila) |
| PAR-4 | Serine/threonine kinase | LKB1 homolog, energy sensing | Symmetric (cortical and cytoplasmic) | High |
| PAR-5 | 14-3-3 protein | Binds phospho-serine/threonine motifs | Symmetric (cortical and cytoplasmic) | High |
| PAR-6 | PDZ domain adaptor protein | Links PKC-3 to PAR-3 and CDC-42 | Anterior cortex | High |
| PKC-3 | Atypical protein kinase C | Phosphorylates PAR-1 and PAR-2 | Anterior cortex | High |
| CDC-42 | Small GTPase | Binds PAR-6, regulates cytoskeleton | Anterior cortex (active form) | High |
The PAR proteins function through a complex network of mutual antagonisms and cooperative interactions that establish and maintain complementary cortical domains [40]. The anterior PAR proteins (aPARs: PAR-3, PAR-6, PKC-3) and posterior PAR proteins (pPARs: PAR-1, PAR-2) form two mutually exclusive cortical domains, while PAR-4 and PAR-5 are uniformly distributed but essential for proper polarization [1].
Molecular Circuitry of PAR Protein Interactions
Hierarchical Assembly of PAR Complexes
The assembly of PAR complexes follows a precise hierarchy that ensures proper spatial organization. Cortical recruitment of aPARs is governed by independent membrane binding of PAR-3 and CDC-42, which synergize to recruit PAR-6 and PKC-3 [40]. PAR-3 recruitment depends on direct binding to membrane phospholipids, self-oligomerization through its N-terminal CR1 domain, and specific protein-protein interactions mediated by its three PDZ domains [40].
The formation of the core anterior complex involves PAR-3 oligomerization creating a membrane-associated scaffold that recruits PAR-6-PKC-3 heterodimers. PAR-6 simultaneously binds to CDC-42-GTP, creating a stable tripartite complex (PAR-3/PAR-6/PKC-3) that constitutes the functional aPAR module [40] [51]. This complex has kinase activity toward downstream substrates, including the pPARs.
Posterior PAR proteins assemble through a different mechanism. PAR-2 localizes to the posterior cortex independently and recruits PAR-1, potentially through direct binding that protects PAR-1 from PKC-3-mediated phosphorylation and displacement [40] [51]. PAR-1 itself is a kinase that phosphorylates PAR-3, reducing its membrane affinity and creating a mutual exclusion system.
Mutual Antagonism and Phosphorylation Networks
The establishment and maintenance of complementary PAR domains relies on mutual antagonism between anterior and posterior PARs:
- Anterior to posterior inhibition: PKC-3 phosphorylates both PAR-1 and PAR-2, reducing their membrane affinity and promoting their dissociation from the cortex [51]. Phosphorylated pPARs are recognized by PAR-5 (14-3-3 proteins), which sequesters them in the cytoplasm or promotes their degradation.
- Posterior to anterior inhibition: PAR-1 phosphorylates PAR-3, disrupting its membrane localization and oligomerization capacity [40] [51]. PAR-2 may also contribute to aPAR displacement through incompletely characterized mechanisms potentially involving regulation of CDC-42 activity.
This mutual inhibition creates a bistable system that reinforces the boundary between anterior and posterior domains once established.
Diagram 1: Core PAR protein interaction network showing mutual antagonism
Integration with Cytoskeletal and Geometric Regulation
Beyond the core phosphorylation network, PAR proteins interface with cytoskeletal elements and respond to geometric cues. CDC-42, part of the aPAR complex, activates the kinase MRCK-1, which in turn activates non-muscle myosin II, creating a gradient of cortical contractility that drives anterior-directed cortical flows [40]. These flows enhance the segregation of aPARs and pPARs into distinct domains.
Geometric factors also influence PAR polarization. The local ratio of membrane surface to cytosolic volume varies along the cell surface, being highest at cell poles and lowest at the midcell [22]. This geometric cue affects the rebinding probability of proteins after detachment, creating preferential sites for domain stabilization. Computational models demonstrate that the length of the aPAR-pPAR interface, combined with the kinetics of phosphorylation-dephosphorylation cycles, favors long-axis polarization in elliptical cells like the C. elegans zygote [22].
Quantitative Analysis of PAR Protein Dynamics
Advanced biophysical techniques including fluorescence recovery after photobleaching (FRAP) and single-molecule imaging have quantified the dynamic behavior of PAR proteins, revealing their kinetic properties and exchange rates.
Table 2: Quantitative Parameters of PAR Protein Dynamics in C. elegans Zygote
| Parameter | Value/Range | Measurement Technique | Biological Significance |
|---|---|---|---|
| aPAR membrane residence time | ~10-20 seconds | FRAP, single-particle tracking | Determines complex stability before exchange |
| pPAR membrane residence time | ~5-15 seconds | FRAP, single-particle tracking | Reflects phosphorylation-mediated turnover |
| Cortical flow velocity | ~0.1-0.2 μm/s | Particle image velocimetry | Drives initial asymmetry establishment |
| Phosphorylation rate (PKC-3 on PAR-1) | ~0.1-1.0 sâ»Â¹ | Kinetic modeling, in vitro assays | Sets timescale for mutual antagonism |
| Dephosphorylation rate (PAR-1 reactivation) | ~0.01-0.1 sâ»Â¹ | Mathematical modeling [22] | Controls rebinding probability after displacement |
| Interface width between domains | ~2-5 μm | Fluorescence intensity profiling | Reflects sharpness of boundary established by mutual inhibition |
These quantitative parameters inform mathematical models that reproduce key features of PAR polarization and predict behaviors in mutant backgrounds. The dynamic exchange of PAR proteins between cytoplasmic and membrane pools is essential for both the establishment and maintenance phases of polarity.
Experimental Methods for Analyzing PAR Protein Function
Genetic Approaches and Mutant Analysis
Traditional genetic screens formed the foundation of PAR protein research. The original par mutants were identified in C. elegans through maternal-effect lethal screens that revealed defects in asymmetric cell division and partitioning of cytoplasmic determinants [1]. Current approaches include:
- RNAi-mediated knockdown: Tissue-specific and timed knockdowns to assess function in later developmental stages including gastrulation.
- CRISPR/Cas9 genome editing: Generation of tagged proteins, point mutations, and tissue-specific knockouts.
- Temperature-sensitive alleles: For temporal control of protein function during specific developmental windows.
Genetic epistasis analyses have established the hierarchical relationships between PAR genes, placing PAR-3, PAR-6, and PKC-3 in the anterior group and PAR-1 and PAR-2 in the posterior group, with PAR-4 and PAR-5 required for both domains.
Live Imaging and Biophysical Analysis
Advanced live imaging techniques enable quantitative analysis of PAR protein dynamics:
Diagram 2: Experimental workflow for quantitative analysis of PAR protein dynamics
Protocol: Fluorescence Recovery After Photobleaching (FRAP) for PAR Protein Dynamics
- Sample Preparation: Generate embryos expressing endogenously tagged PAR proteins (e.g., PAR-2::GFP) using CRISPR/Cas9 genome editing.
- Image Acquisition: Use a confocal microscope with 488nm laser line, high-sensitivity detectors, and environmental control (20°C).
- Photobleaching: Define a region of interest (ROI) at the cortex and bleach with high-intensity 488nm laser (100% power, 5 iterations).
- Recovery Imaging: Acquire images at 1-second intervals for 2-5 minutes with low laser power (1-5%) to minimize phototoxicity.
- Data Analysis:
- Measure fluorescence intensity in bleached ROI and normalize to background and pre-bleach levels.
- Fit recovery curve to exponential function: I(t) = Iâ‚€ + I_max(1 - e^(-Ï„t))
- Calculate half-time of recovery (tâ‚/â‚‚ = ln(2)/Ï„) and mobile fraction.
Protocol: Computational Modeling of PAR Protein Interactions
- Define Reaction Network: Specify known biochemical interactions (phosphorylation, membrane binding, complex formation).
- Formulate Equations: Convert reactions to partial differential equations describing concentration changes in space and time.
- Parameter Estimation: Use experimental data (FRAP, concentrations, domain sizes) to constrain parameter values.
- Numerical Simulation: Solve equations using finite element methods on realistic cell geometries.
- Model Validation: Compare simulation predictions with experimental observations in wild-type and mutant backgrounds.
Research Reagent Solutions for PAR Protein Studies
Table 3: Essential Research Reagents for PAR Protein Investigations
| Reagent Category | Specific Examples | Application | Technical Considerations |
|---|---|---|---|
| Antibodies | Anti-PAR-3 (polyclonal), Anti-PAR-1 (monoclonal) | Immunofluorescence, Western blotting | Verify specificity in par mutant backgrounds |
| Fluorescent protein tags | GFP, mNeonGreen, HALO-tag | Live imaging, pulse-chase experiments | Endogenous tagging preferred over transgenes |
| Mutant strains | par-1(RNAi), par-3(zu310) | Functional analysis | Use balanced stocks for lethal mutations |
| Biochemical reagents | CDC-42(GTPγS), PKC-3 inhibitor | In vitro assays | Validate activity in orthogonal assays |
| Mathematical models | Reaction-diffusion framework [13] | Theoretical predictions | Implement parameter sensitivity analysis |
| Geometric manipulation | Microfluidic compression devices | Altered cell shape studies | Control for activation of stress responses |
Adaptation of PAR Circuitry for Gastrulation and Beyond
During C. elegans gastrulation, PAR proteins function in multiple cell types beyond the initial embryonic divisions. The core circuitry is maintained but connected to different upstream regulators and downstream effectors appropriate for each cellular context.
In gastrulating cells, PAR proteins integrate with mesodermal and endodermal specification pathways, guiding cell ingression and migration. The fundamental mechanism of mutual antagonism between aPARs and pPARs is preserved, but the initial symmetry-breaking cues may originate from cell-cell contacts rather than sperm-derived signals [1].
Recent research has revealed how the PAR network maintains robustness through:
- Feedback amplification: Reciprocal interactions between PAR proteins and the cytoskeleton create positive feedback loops that reinforce polarity.
- Spatial sensing: Geometric cues including membrane curvature and local surface-to-volume ratios influence polarization sites [22].
- Stoichiometric balancing: The relative abundances of aPARs and pPARs are regulated to maintain system bistability.
The conservation of PAR protein function across diverse cell types and organisms highlights their fundamental role as a tunable polarity module that can be adapted through regulatory evolution while maintaining core circuit integrity.
The PAR protein network represents a premier example of how a core biochemical module can be evolutionarily tuned for diverse polarizing cues while maintaining its fundamental operational principles. The molecular circuitry of mutual antagonism, hierarchical complex assembly, and integration with cytoskeletal elements provides a robust foundation for cellular polarization that can be adapted to different developmental contexts.
Future research directions with particular relevance for drug development include:
- Targeting PAR protein interactions in diseases of epithelial organization (cancer, developmental disorders)
- Exploiting the geometric sensing properties of the PAR network for tissue engineering applications
- Developing small molecule inhibitors of specific PAR protein interactions for therapeutic intervention
- Engineering synthetic polarity systems based on PAR network principles
The continued investigation of PAR protein function in C. elegans gastrulation and other developmental contexts will undoubtedly yield further insights into how conserved molecular circuits are adapted to generate cellular diversity, with broad implications for basic biology and translational applications.
Conclusion
The study of PAR proteins in C. elegans gastrulation reveals a powerful paradigm where a deeply conserved molecular module is repurposed to orchestrate complex morphogenetic events. The core circuitry, built on mutual antagonism and dynamic exchange, is remarkably versatile, translating transient cues into stable cellular asymmetries that direct actomyosin-driven apical constriction and cell ingression. Future research must bridge the gap between the well-characterized upstream polarity network and its downstream effectors, precisely defining how PAR proteins command the cytoskeletal remodeling that powers cell movement. Furthermore, the emerging roles of PAR proteins in organizing non-centrosomal microtubules suggest an even broader regulatory scope. For biomedical research, understanding how these fundamental polarity mechanisms are deployed, adapted, or disrupted provides critical insights into human developmental disorders and disease processes like cancer metastasis, where the control of cell polarity and movement is paramount.
References
- 1. The PAR Proteins: Fundamental Players in Animal Cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2964935/]
- 2. CDC-42 Interactions with Par Proteins Are Critical for Proper ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7565983/]
- 3. Polarity and cell fate specification in the control of C. elegans ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2929021/]
- 4. PAR proteins diffuse freely across the anterior–posterior ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3087016/]
- 5. Quantitative perturbation-phenotype maps reveal nonlinear ... [https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3002437]
- 6. Geometric cues stabilise long-axis polarisation of PAR protein ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6985163/]
- 7. C. elegans Brat homologs regulate PAR protein-dependent ... [https://www.sciencedirect.com/science/article/pii/S0012160608010464]
- 8. High-throughput imaging and quantitative analysis ... [https://elifesciences.org/articles/78743]
- 9. Targeting the chromosome partitioning protein ParA in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2980951/]
- 10. A balance between antagonizing PAR proteins specifies ... [https://www.sciencedirect.com/science/article/pii/S2211124721007026]
- 11. Apical PAR protein caps orient the mitotic spindle in C. ... [https://www.sciencedirect.com/science/article/pii/S0960982223011491]
- 12. Multiple pathways for reestablishing PAR polarity in C. ... [https://www.sciencedirect.com/science/article/pii/S0012160623000878]
- 13. Modeling the Establishment of PAR Protein Polarity in ... [https://www.sciencedirect.com/science/article/pii/S0006349508785928]
- 14. Gastrulation in C. elegans - WormBook - NCBI Bookshelf [https://www.ncbi.nlm.nih.gov/books/NBK19716/]
- 15. FRAP: A Powerful Method to Evaluate Membrane Fluidity ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8328621/]
- 16. Visualizing and quantifying molecular and cellular ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9339300/]
- 17. Balancing reaction-diffusion network for cell polarization ... [https://elifesciences.org/articles/96421]
- 18. Balancing reaction-diffusion network for cell polarization ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12283076/]
- 19. Spatial modeling algorithms for reactions and transport in ... [https://www.nature.com/articles/s43588-024-00745-x]
- 20. Agent-based Monte Carlo simulations for reaction-diffusion ... [https://arxiv.org/html/2505.18145v2]
- 21. The PAR Proteins: Fundamental Players in Animal Cell ... [https://www.sciencedirect.com/science/article/pii/S1534580707003851]
- 22. Geometric cues stabilise long-axis polarisation of PAR ... [https://www.nature.com/articles/s41467-020-14317-w]
- 23. Cell polarity and gastrulation in C. elegans [https://pubmed.ncbi.nlm.nih.gov/11807031/]
- 24. Research – Nance Lab – UW–Madison [https://nancelab.crb.wisc.edu/research/]
- 25. Geometric cues stabilise long-axis polarisation of PAR ... [https://pubmed.ncbi.nlm.nih.gov/31988277/]
- 26. Geometric cues stabilise long-axis polarisation of PAR ... [https://www.theorie.physik.uni-muenchen.de/lsfrey/research/biological_physics/18_10_25/index.html]
- 27. Modeling Parkinson's Disease in C. elegans - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC5836411/]
- 28. Balancing cell polarity PARts through dephosphorylation - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9486083/]
- 29. Epidermal PAR-6 and PKC-3 are essential for larval ... [https://elifesciences.org/articles/62067]
- 30. . C -3 and elegans -6 are required for apicobasal asymmetries... PAR PAR [https://pubmed.ncbi.nlm.nih.gov/13129846/]
- 31. PAR proteins regulate maintenance-phase myosin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5531737/]
- 32. Gastrulation: PARtaking of the Bottle [https://www.sciencedirect.com/science/article/pii/S0960982203001556]
- 33. fzr-1 and lin-35/Rb function redundantly to control cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC155341/]
- 34. Quantitative high-throughput analysis of synthetic genetic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4443778/]
- 35. Quantitative Analysis and Modeling Probe Polarity ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4336357/]
- 36. The auxin-inducible degradation (AID) system ... - PubMed [https://pubmed.ncbi.nlm.nih.gov/26552885/]
- 37. Targeting endogenous proteins for spatial and temporal ... [https://www.sciencedirect.com/science/article/pii/S266616672200908X]
- 38. The auxin-inducible degron 2 (AID2) system ... - PubMed [https://pubmed.ncbi.nlm.nih.gov/34865044/]
- 39. Protocol for auxin-inducible protein degradation in C. ... [https://www.sciencedirect.com/science/article/pii/S2666166724002983]
- 40. The PAR proteins: from molecular circuits to dynamic self ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5665476/]
- 41. Elaborating polarity: PAR proteins and the cytoskeleton - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3035085/]
- 42. PAR proteins and the cytoskeleton: a marriage of equals [https://www.sciencedirect.com/science/article/abs/pii/S0955067405001882]
- 43. Par proteins and neuronal polarity - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC3153582/]
- 44. Apical PAR complex proteins protect against programmed ... [https://elifesciences.org/articles/64437]
- 45. Polarity protein Par6: Unraveling its mechanisms in tumor ... [https://www.sciencedirect.com/science/article/abs/pii/S0898656825005960]
- 46. Caenorhabditis elegans Gastrulation: A Model for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7017025/]
- 47. Non-centrosomal epidermal microtubules act in parallel to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6514414/]
- 48. NOCA-1 functions with γ-tubulin and in parallel to Patronin to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4608005/]
- 49. PTRN-1 (CAMSAP) and NOCA-2 (NINEIN) are required for ... [https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3001855]
- 50. Wnt/Frizzled Signaling Controls C. elegans Gastrulation by ... [https://www.sciencedirect.com/science/article/pii/S0960982206022032]
- 51. CDC-42 Interactions with Par Proteins Are Critical for ... [https://www.mdpi.com/2073-4409/9/9/2036]