Illuminating Gastrulation: Controlling Cell Internalization with Optogenetics
This article explores the transformative application of optogenetics to control cell internalization processes during gastrulation, a pivotal stage in embryonic development.
Illuminating Gastrulation: Controlling Cell Internalization with Optogenetics
Abstract
This article explores the transformative application of optogenetics to control cell internalization processes during gastrulation, a pivotal stage in embryonic development. We provide a foundational overview of gastrulation and the limitations of traditional study methods, then detail the current optogenetic toolkit—including light-sensitive protein domains and gene expression systems—for precise spatiotemporal manipulation. The content further addresses key methodological challenges, such as light delivery and phototoxicity, and validates the approach by comparing it with pharmacological and genetic techniques. Aimed at researchers, scientists, and drug development professionals, this resource synthesizes cutting-edge findings to guide the use of optogenetics in developmental biology and regenerative medicine.
Gastrulation and the Need for Precision: Why Optogenetics is a Game-Changer
Gastrulation is a pivotal stage in early embryonic development during which a single-layered blastula reorganizes into a multi-layered structure, establishing the foundational body plan. This process involves elaborate cell movements that form the three primary germ layers: the ectoderm (which gives rise to the skin and nervous system), the mesoderm (precursor to muscles, bones, and the heart), and the endoderm (which forms the gut and associated organs) [1]. A central outcome of gastrulation is the establishment of the three body axes—head/tail (anterior-posterior), front/back (dorsal-ventral), and left/right—that create the spatial blueprint for all subsequent tissue and organ development [2] [3]. Disruptions during this finely tuned process can lead to developmental defects, underscoring the need for precise experimental models to deconstruct its underlying mechanisms [1].
Historically, the study of human gastrulation has been constrained by ethical considerations and technical hurdles, as it occurs deep within the uterus shortly after implantation [2] [3]. Recent advances, however, have ushered in a new era of research. The integration of stem cell technology, synthetic embryo models, and optogenetics is now providing unprecedented insights. A paradigm shift is underway, moving beyond the purely biochemical understanding of gastrulation to one that incorporates the essential role of physical forces and mechanical competence in guiding cell fate and tissue organization [2]. This Application Note details these mechanistic insights and provides practical protocols for investigating the control of cell internalization during gastrulation.
Core Principles: Beyond Biochemical Signals
The prevailing view of gastrulation has been dominated by the actions of morphogens, such as BMP4, Nodal, and WNT, which instruct cell behavior through concentration gradients. While these signals are indispensable, recent research reveals they are not sufficient. A complete picture of gastrulation must account for the interplay between biochemical cues and the physical microenvironment.
The Emergence of Mechanical Forces
Evidence now confirms that mechanical forces are a critical partner to molecular signals in breaking embryonic symmetry and driving gastrulation. In human gastrula models, activating the BMP4 protein with light (optogenetics) was insufficient to trigger full gastrulation in low-tension environments. The process robustly proceeded only when BMP4 activation occurred under specific mechanical confinement that induced cellular tension [2]. This tension is sensed by the mechanosensory protein YAP1, which fine-tunes downstream biochemical pathways like WNT and Nodal. Nuclear YAP1 appears to act as a molecular brake on gastrulation, ensuring the transformation does not initiate prematurely. This suggests cells must achieve a state of "mechanical competence"—being both chemically primed and physically primed—to undergo gastrulation successfully [2] [3].
Novel Modes of Collective Cell Migration
As cells ingress and migrate to form the germ layers, their movement is not merely a solitary endeavor. In chick embryos, mesoderm cells emerging from the primitive streak exhibit a novel form of collective migration. Rather than moving as discrete individuals, they form a dynamic, constantly reorganizing 3D meshwork structure [4] [5]. This collective behavior is mediated by transient, N-cadherin-mediated cell-cell adhesion. Disrupting N-cadherin function reduces the directionality and speed of tissue progression without affecting individual cell speed, highlighting that the collective supracellular structure, not just single-cell motility, is key to efficient migration [5]. Agent-based modeling suggests that cell elongation, adhesion strength, and cell density are key parameters governing this meshwork formation [4] [5].
Application Note: An Optogenetic Protocol for Controlling Cell Internalization
This section provides a detailed methodology for using optogenetics to control Nodal signaling, a key pathway directing cell internalization and fate specification during gastrulation. The protocol is adapted from recent work in zebrafish embryos [6].
Experimental Workflow for Optogenetic Patterning of Nodal Signaling
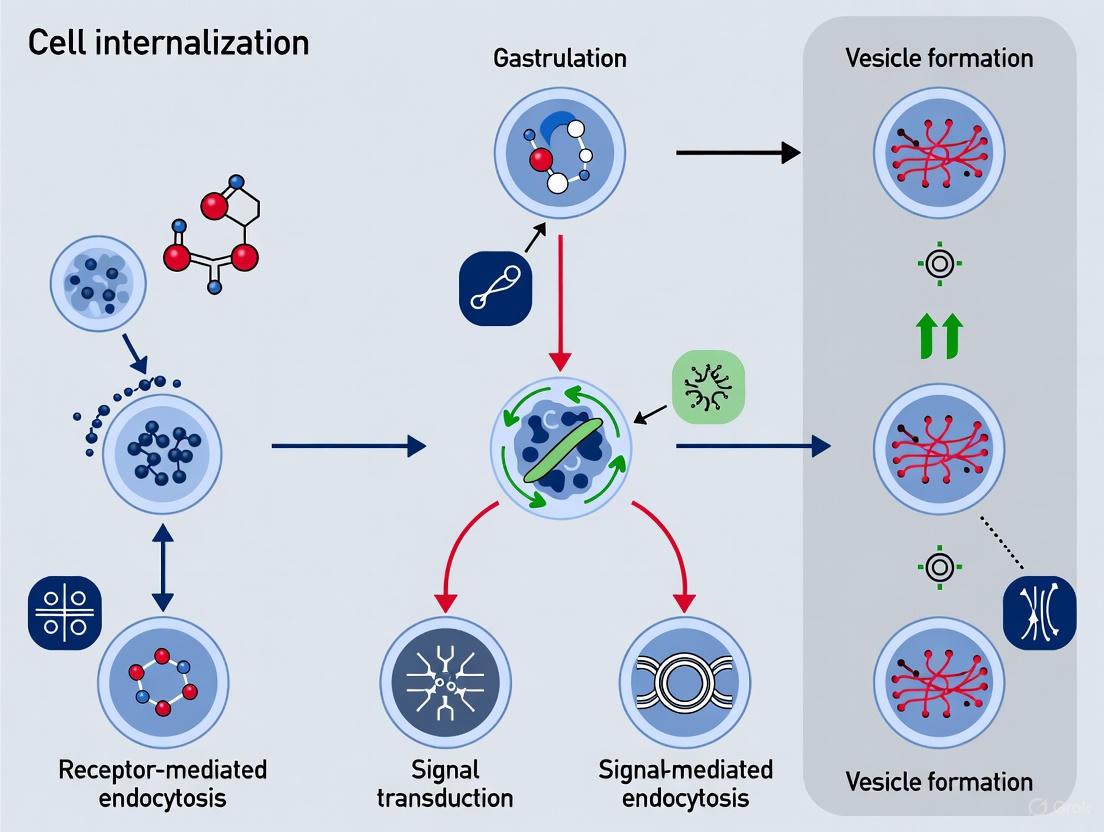
The following diagram outlines the core workflow for controlling cell internalization using an optogenetic system.
Detailed Experimental Procedures
Reagent Preparation: optoNodal2 System
The improved optoNodal2 reagent is crucial for high-fidelity spatial patterning. It consists of two components:
- CRY2-acvr1b (Type I Receptor): Fused to the CRY2 photodimerizing domain.
- CIB1N-acvr2b (Type II Receptor): Fused to the CIB1N domain and engineered with a cytosolic sequestration signal to minimize dark activity.
Procedure:
- Plasmids: Obtain plasmids encoding the CRY2-acvr1b and CIB1N-acvr2b fusion proteins.
- mRNA Synthesis: Linearize the plasmid templates and synthesize capped mRNA in vitro using a commercial mRNA synthesis kit.
- Purification: Purify the synthesized mRNA using a standard phenol-chloroform extraction and isopropanol precipitation protocol. Resuspend the mRNA pellet in nuclease-free water.
- Quantification and Storage: Measure mRNA concentration via spectrophotometry, aliquot, and store at -80°C. Avoid repeated freeze-thaw cycles.
Embryo Microinjection and Preparation
- Zebrafish Embryos: Collect one-cell stage zebrafish embryos and align them on an agarose injection plate.
- Microinjection: Prepare an injection mix containing both CRY2-acvr1b and CIB1N-acvr2b mRNAs (25-50 pg of each per embryo). Backload the mix into a glass capillary needle and microinject into the yolk or cell cytoplasm of one-cell stage embryos.
- Dark Incubation: Post-injection, immediately transfer the embryos to a light-tight container. Incubate in the dark at 28.5°C until the desired developmental stage (e.g., shield stage for internalization studies) is reached. All subsequent steps before fixation must be performed under dim red light, which does not activate the CRY2/CIB1N pair.
Optogenetic Patterning and Live Imaging
- Mounting: At the appropriate stage, dechorionate the embryos and embed in a low-melting-point agarose within a glass-bottom imaging dish.
- Light Patterning System: Use a custom ultra-widefield or commercial patterned illumination microscope system capable of projecting user-defined blue light (e.g., 488 nm laser) patterns onto the sample.
- Stimulation Protocol: To induce Nodal signaling in a specific spatial pattern (e.g., a gradient or a sharp boundary), project the corresponding light pattern onto the embryos. A typical stimulation might use light intensities of 0.1-1 mW/mm² for durations of 15-60 minutes.
- Live Imaging: Simultaneously image the embryos using a low-intensity red fluorescent channel (e.g., RFP or Cy5) to track cell movements during internalization. To monitor immediate pathway activation, you can use a transgenic line or antibody staining for phosphorylated Smad2 (pSmad2), which translocates to the nucleus upon Nodal activation.
Post-Stimulation Analysis
- Fixation: At the end of the experiment, fix embryos in 4% paraformaldehyde (PFA) for 2 hours at room temperature.
- Immunostaining: Perform standard immunostaining protocols for pSmad2 to visualize the spatial domain of Nodal signaling activation and for markers of mesendodermal lineages (e.g., Sox32 for endoderm).
- In Situ Hybridization: To assess downstream gene expression, perform whole-mount in situ hybridization for early Nodal target genes (e.g., gsc, ntl).
- Image Analysis: Use image analysis software to quantify the extent of cell internalization, the correlation between the light pattern and pSmad2 nuclear localization, and the expression domains of target genes.
Signaling Pathway and Experimental Logic
The mechanistic logic of the optoNodal2 system and its integration with mechanical forces is summarized below.
Key Quantitative Data from Gastrulation Studies
Table 1: Quantitative Parameters of Mesoderm Cell Migration in Chick Gastrulation [5]
| Parameter | Measurement | Experimental Context |
|---|---|---|
| Individual Cell Speed | 2.3 - 4.0 µm/min | Measured from 3D trajectories of H2B-eGFP labeled mesoderm cells in chick embryos. |
| Directionality (Persistence) | 0.45 - 0.7 (for 20 min tracks) | Ratio of start-to-end distance to total path length; lower values indicate more frequent turning. |
| Z-Position Change | ~20 µm (peak, over 60 min) | Frequent movement through 1-2 cell lengths in the Z-axis (between ectoderm and endoderm). |
| Migration Direction | Anterior-lateral (Anterior/Middle streak); Lateral (Posterior streak) | Consistent with previously described mesoderm migration pathways. |
Table 2: Key Parameters for Mesoderm Meshwork Formation from an Agent-Based Model [4] [5]
| Model Parameter | Impact on Meshwork Formation |
|---|---|
| Cell Elongation | Increased elongation promotes the extension of cellular processes that form the meshwork connections. |
| Cell-Cell Adhesion | Stronger N-cadherin mediated adhesion stabilizes transient connections, enabling collective movement. |
| Cell Density | Optimal density is required to provide sufficient contact points for a connected network to form. |
The Scientist's Toolkit: Essential Research Reagents and Models
Table 3: Key Reagent Solutions for Gastrulation and Cell Internalization Research
| Tool / Reagent | Function / Application | Example Use in Gastrulation Research |
|---|---|---|
| Optogenetic Receptors (e.g., optoNodal2) | High spatiotemporal control of specific signaling pathways. | Precisely patterning Nodal signaling to study its role in directing mesendoderm internalization [6]. |
| Synthetic Embryos (Gastruloids) | Ethically accessible models of early development from human stem cells. | Studying human mesoderm migration and differentiation in a controlled, 2D or 3D environment [1] [7]. |
| Micropatterned Substrates | Control over tissue geometry and emergent mechanical forces. | Investigating how confinement-induced tension regulates symmetry breaking and germ layer specification [2]. |
| Line-Scan Brillouin Microscopy (LSBM) | Non-invasive, volumetric mapping of cell material properties (longitudinal modulus). | Revealing rapid, spatially varying mechanical changes in Drosophila mesoderm cells during invagination [8]. |
| Agent-Based Theoretical Models | Computational simulation of emergent cell behaviors from simple rules. | Testing the sufficiency of parameters like adhesion and cell shape in forming the observed mesoderm meshwork [5]. |
| 2-Methyl-1,1-bis(2-methylpropoxy)propane | 2-Methyl-1,1-bis(2-methylpropoxy)propane|C12H26O2 | 2-Methyl-1,1-bis(2-methylpropoxy)propane (C12H26O2) is a high-purity solvent for advanced research. This product is For Research Use Only. Not for diagnostic or personal use. |
| 3-Bromo-2-hydroxypropanoic acid | 3-Bromo-2-hydroxypropanoic acid, CAS:32777-03-0, MF:C3H5BrO3, MW:168.97 g/mol | Chemical Reagent |
Discussion and Future Directions
The integration of optogenetics, advanced imaging, and engineered model systems is fundamentally reshaping our understanding of gastrulation. The evidence is clear: biochemical signaling and mechanical forces are inextricably linked in guiding the formation of the body axes. The concept of "mechanical competence" provides a useful framework for understanding why certain cell populations are primed to respond to differentiation signals while others are not [2] [3].
Future research will likely focus on the existence of a "mechanical organizer"—a hypothesized force-based counterpart to classical signaling centers like the Spemann organizer [2]. Furthermore, the application of more sophisticated optogenetic tools to control multiple pathways simultaneously (e.g., Nodal, BMP, WNT) will allow researchers to reconstruct and test the complex signaling networks that orchestrate development. Finally, extending the culture duration and structural complexity of in vitro gastruloid models will be crucial for moving from studying the initial breaking of symmetry to understanding later stages of organogenesis [1] [7]. These advances, detailed in the protocols herein, will not only illuminate fundamental biology but also pave the way for innovations in regenerative medicine and the treatment of infertility and developmental disorders.
Application Notes
AN-1: Quantitative Profiling of Cell Internalization During Gastrulation
Purpose: This application note details a standardized method for quantifying the key cellular events of gastrulation, establishing a baseline for assessing perturbations by optogenetic tools.
Background: Gastrulation involves the coordinated internalization of specific progenitor cells to form the embryonic germ layers. In C. elegans, a total of 66 cells internalize in a precise spatiotemporal sequence, beginning with the two endodermal precursors (Ea/Ep) at the 28-cell stage [9] [10]. The primary morphogenetic module for this internalization is actomyosin-driven. Internalizing cells exhibit pulsatile, apical contractions of an actomyosin network, while surrounding cells form centripetal extensions that converge into multicellular rosettes, sealing over the internalizing cells in a process distinct from actin purse-string-mediated closure [10]. This module is recurrently deployed throughout gastrulation, demonstrating significant plasticity to accommodate variations in cell number and size [10].
Key Quantitative Parameters: The following parameters, derived from wild-type C. elegans and Drosophila studies, should be measured to establish a normative profile [9] [11] [10].
Table 1: Key Quantitative Parameters of Gastrulation
| Parameter | Experimental System | Typical Wild-type Value / Observation | Measurement Technique |
|---|---|---|---|
| Total Gastrulating Cells | C. elegans | 66 cells [9] | 4D live imaging & cell lineage tracing |
| Onset of Internalization | C. elegans | 28-cell stage [9] | 4D live imaging |
| Core Morphogenetic Module | C. elegans | Actomyosin-based apical contractility & rosette formation [10] | High-resolution timelapse microscopy |
| Temporal Sampling Requirement | Drosophila | ≤ 45 seconds [11] | 4D imaging (2PEF microscopy) |
| Spatial Resolution Requirement | Drosophila | 0.5 μm (x,y) x 1.0 μm (z) [11] | 4D imaging (2PEF microscopy) |
| Plasticity of Process | C. elegans | Adapts to internalize ectopic endodermal cells [10] | Genetic perturbation (e.g., pop-1 RNAi) |
AN-2: Validating Optogenetic Perturbations of Internalization
Purpose: To provide a framework for using the quantitative profiles in AN-1 to assess the efficacy and precision of light-controlled interventions targeting actomyosin contractility or cell polarity.
Validation Workflow:
- Spatiotemporal Targeting: Express the optogenetic actuator (e.g., a photoactivatable RhoGEF) in a specific cell lineage.
- Stimulation Protocol: Apply light patterns to defined embryonic regions with precise timing and duration.
- Phenotypic Quantification: Acquire 4D image datasets of perturbed embryos and measure the parameters in Table 1.
- Analysis: Compare the internalization dynamics (timing, rosette formation, cell trajectories) against the wild-type baseline. Successful perturbation is indicated by significant, localized deviations, such as delayed internalization or failure of rosette closure in the targeted cells.
Experimental Protocols
Protocol 1: 4D Live Imaging and Quantitative Analysis of Gastrulation
This protocol is adapted from established methods for Drosophila and integrates principles from C. elegans studies [9] [11] [10].
I. Embryo Preparation and Mounting
- Fluorescent Labeling: Generate embryos with ubiquitous nuclear fluorescent labeling. A histone fusion (e.g., H2A::GFP) is superior to a nuclear localization sequence (NLS) fusion as it remains associated with chromosomes during mitosis [11].
- Dechorionation: Mechanically or chemically remove the chorion.
- Mounting: Adhere embryos to a coverslip using a thin layer of glue. For imaging, place the mounted embryos in a chamber with water or appropriate medium. Critical: The mounting setup must immobilize the embryo to prevent motion artifacts and be optically adapted for high-resolution microscopy [11].
II. 4D Image Acquisition via Multiphoton Microscopy
- Microscope Setup: Use a two-photon excited fluorescence (2PEF) microscope with a water-immersion objective (e.g., 40x, 1.1 NA, large working distance) to maximize imaging depth [11].
- Acquisition Parameters:
- Field of View: ~200 μm x 200 μm to capture the relevant cell populations.
- Spatial Sampling: 0.5 μm x 0.5 μm x 1.0 μm (x, y, z) to ensure accurate nuclear segmentation.
- Temporal Sampling: Acquire z-stacks every 45 seconds or less to faithfully track cell movements.
- Excitation Wavelength: ~940 nm for efficient GFP excitation with low phototoxicity and background.
- Laser Power: Keep mean power below 30 mW to minimize photodamage. Include a resting time (e.g., 10 s) between successive z-stack acquisitions [11].
III. 3D Cell Tracking and Data Registration
- Nuclear Segmentation: Use commercial (e.g., Imaris) or custom software to identify and segment nuclei in 3D for each time point.
- Cell Tracking: Link segmented nuclei across time points to generate 3D trajectories for each cell. Manual correction may be necessary.
- Spatial Registration: Correct for global embryo movements (e.g., rotation, drift) using a segmented-based registration algorithm. Align tracking data to a biologically relevant coordinate system (e.g., cylindrical coordinates for a Drosophila embryo) [11].
- Temporal Registration: Synchronize the start of image sequences across multiple embryos based on a conserved biological landmark (e.g., the onset of germband extension in Drosophila) [11].
IV. Quantitative Analysis of Cell Behavior
- Movement Decomposition: Analyze cell trajectories within the registered coordinate system to decompose complex 3D movements into directional components (e.g., radial, angular, axial).
- Internalization Timing: Score the time at which each target cell becomes fully covered by neighboring cells.
- Collective Migration Analysis: Perform statistical analysis on cell trajectories to quantify the collective nature of migration, for example, by calculating correlation coefficients between the starting and ending angular positions of mesoderm cells [11].
Protocol 2: Genetic Perturbation of Cell Fate and Analysis of Internalization Plasticity
This protocol is based on experiments in C. elegans demonstrating the adaptability of the internalization module [10].
- Perturbation of Cell Fate:
- Use RNA interference (RNAi) against the pop-1 gene (a TCF transcription factor) to transform mesodermal precursor cells into endodermal fate.
- Live Imaging of Phenotype:
- Mount the resulting embryos and perform 4D imaging as described in Protocol 1.
- Analysis of Morphogenetic Plasticity:
- Confirm that the ectopic endoderm cells accumulate apical NMY-2::GFP (non-muscle myosin II).
- Score the ability of surrounding cells to form centripetal extensions and multicellular rosettes that seal over the ectopic endoderm cells. In wild-type embryos, these surrounding cells would not extend over other cells at this time [10].
Visualizing Gastrulation Mechanisms and Workflows
Diagram 1: Gastrulation Internalization Mechanism
Diagram 2: Quantitative Imaging & Analysis Workflow
Research Reagent Solutions
Table 2: Essential Reagents for Gastrulation Research
| Reagent / Material | Function / Application | Example / Note |
|---|---|---|
| H2A::GFP Transgenic Line | Ubiquitous nuclear labeling for robust 4D segmentation and tracking, even during mitosis. | Superior to NLS-GFP lines [11]. |
| klarsicht (klar) Mutant | Alters lipid droplet distribution, reducing light scattering and improving imaging depth. | Useful in Drosophila; viable homozygous mutants [11]. |
| NMY-2::GFP Reporter | Visualizes the dynamics of non-muscle myosin II during apical constriction. | Critical for imaging actomyosin contractility in C. elegans [9] [10]. |
| RNAi Constructs (e.g., pop-1) | Genetically perturbs cell fate to test the plasticity of morphogenetic modules. | Used to create ectopic endoderm in C. elegans [10]. |
| par-3/par-6 Mutants | Disrupts apicobasal polarity to investigate its role in directing contractile forces. | Used in C. elegans to study polarity in internalization [9]. |
Optogenetics is a powerful technique that enables the control of protein function and cellular signaling with high spatiotemporal precision using light [12]. By rewiring signaling pathways to respond to light, researchers can effectively convert photons into morphogen signals, unlocking a level of control over developmental processes that cannot be achieved with traditional genetic manipulations [6]. This approach has proven particularly transformative for studying fundamental biological processes where precise timing and spatial localization are critical, such as during embryonic development.
A crucial application of optogenetics lies in deciphering how spatial patterns of signaling activity guide embryonic development. Embryos transmit instructions to their cells using concentration-dependent signaling cues called morphogens, which convey positional information to activate appropriate developmental programs [6]. Testing quantitative theories of how morphogens organize development requires the ability to systematically manipulate these spatial and temporal patterns of signaling activity. Optogenetic tools have emerged as a promising strategy for this agile and precise control over developmental gene expression and signaling, allowing investigators to create arbitrary morphogen signaling patterns in time and space to rigorously test specific hypotheses [6].
Essential Research Tools and Reagents
The successful implementation of optogenetic control in biological research relies on a specialized toolkit of reagents and instrumentation. The table below summarizes the core components required for optogenetic investigations of tissue morphogenesis.
Table 1: Research Reagent Solutions for Optogenetic Control of Tissue Mechanics
| Reagent Category | Specific Examples | Function and Application |
|---|---|---|
| Optogenetic Actuators | Cry2/CIB1N fused receptors [6], LOV domain fusion proteins [6] | Light-sensitive heterodimerizing protein pairs that bring signaling components together upon illumination |
| Model Organisms | Drosophila melanogaster (fruit fly) [12], Danio rerio (zebrafish) [13] [6] | Genetically tractable translucent organisms ideal for in vivo light manipulation and imaging |
| Optical Instrumentation | Light-sheet microscopes [13] [14], Holographic patterning systems [13] | Enable precise light delivery for optogenetic activation and high-speed functional imaging |
| Functional Reporters | Genetically encoded calcium indicators (GECIs) [14], Fluorescently tagged proteins [12] | Report cellular activity and protein localization in response to optogenetic perturbation |
Optogenetic Control of Nodal Signaling in Zebrafish Gastrulation
Background and Biological Significance
A prominent application of optogenetics in developmental biology involves controlling Nodal signaling, a TGF-β family morphogen that organizes mesendodermal patterning in vertebrate embryos [6]. In zebrafish, a Nodal signaling gradient establishes a gradient of cell motility and adhesiveness that is critical for ordered cell internalization at the onset of gastrulation [6]. Higher Nodal exposure directs cells toward endodermal fates, while lower levels direct cells to mesodermal fates, making precise control of this signaling pathway essential for understanding the fundamental mechanisms of germ layer formation and tissue internalization.
The OptoNodal2 Reagent System
Recent research has developed an improved optogenetic system called optoNodal2 for creating designer Nodal signaling patterns in live zebrafish embryos [6]. This system represents a significant advancement over first-generation tools, with enhanced dynamic range and improved response kinetics without sacrificing performance. The molecular engineering behind optoNodal2 involves fusing Nodal receptors to the light-sensitive heterodimerizing pair Cry2/CIB1N, with additional sequestration of the type II receptor to the cytosol to minimize background activity [6]. This optimized configuration eliminates dark activity and improves response kinetics, crucial requirements for achieving biologically relevant spatial patterning.
Table 2: Quantitative Performance Metrics of OptoNodal2 System
| Performance Parameter | First-Generation optoNodal | Enhanced optoNodal2 | Biological Impact |
|---|---|---|---|
| Dark Activity | Significant background signaling [6] | Negligible background activity [6] | Enables precise control of basal signaling state |
| Activation Kinetics | Slow response limited by LOV domain dissociation [6] | Fast activation and deactivation [6] | Allows manipulation of dynamic signaling processes |
| Spatial Resolution | Limited patterning capability [6] | Subcellular precision [6] | Supports creation of complex signaling patterns |
| Throughput | Single embryo manipulation [6] | Parallel patterning in up to 36 embryos [6] | Enables high-throughput experimental design |
Experimental Protocol: Optogenetic Patterning of Nodal Signaling
The following protocol describes the essential steps for implementing optogenetic control of Nodal signaling in zebrafish embryos, based on established methodologies [6]:
Sample Preparation and Mounting
- Generate transgenic zebrafish embryos expressing the optoNodal2 construct.
- At the appropriate developmental stage (typically prior to gastrulation), mount embryos in agarose-filled glass-bottom dishes, ensuring proper orientation for light delivery.
- Maintain physiological conditions (28.5°C) throughout the experiment.
Optical System Configuration
- Employ an ultra-widefield microscopy platform capable of parallel light patterning across multiple embryos.
- Configure illumination parameters: use blue light (∼450-488 nm) for Cry2/CIB1N activation with appropriate intensity and exposure patterns.
- Program desired spatial light patterns (gradients, stripes, or arbitrary shapes) using computer-generated holography or digital mirror devices.
Light Patterning and Live Imaging
- Apply patterned illumination to embryos according to experimental design, controlling both spatial distribution and temporal profile (pulsing, oscillations, etc.).
- Simultaneously image downstream responses using fluorescent reporters of Nodal signaling activity (e.g., Smad2 translocation, target gene expression).
- For analyzing cell internalization behaviors, perform time-lapse imaging of gastrulation movements.
Functional Validation and Analysis
- Fix embryos at specific timepoints and perform in situ hybridization for key mesendodermal marker genes (e.g., sox32, gsc).
- Quantify the spatial domains of gene expression and correlate with the applied light patterns.
- Analyze cell tracking data to quantify internalization movements in response to patterned Nodal signaling.
Optogenetic Control of Tissue Mechanics in Drosophila
Background and Biological Significance
Complementary approaches in Drosophila embryogenesis demonstrate how optogenetics can directly control tissue mechanics during morphogenesis. The development of optogenetic methods to either increase or decrease cell contractility has enabled precise analysis of the interplay between cell-cell interactions, tissue geometry, and force transmission during critical events like gastrulation [12]. These techniques allow researchers to manipulate the actomyosin networks that generate mechanical forces for tissue shaping, providing unprecedented insight into how mechanical processes are regulated in space and time.
Experimental Protocol: Modifying Cell Contractility with Light
The following protocol describes the methodology for optogenetic control of tissue mechanics during Drosophila embryonic development [12]:
Sample Preparation and Genetics
- Generate Drosophila embryos expressing optogenetic actuators targeting Rho GTPase signaling or directly engineering actomyosin network components.
- Collect age-matched embryos (0-3 hours old) and dechorionate using standard protocols.
- Mount embryos in halocarbon oil on gas-permeable membrane dishes for optimal imaging and light delivery.
Optogenetic Illumination
- Configure a confocal or light-sheet microscope for simultaneous optogenetic activation and imaging.
- For Rho signaling activation, use blue light illumination (∼458-488 nm) with spatial patterns matching the embryonic regions of interest.
- Control illumination intensity and duration to achieve desired levels of contractility modulation.
Live Imaging of Morphogenetic Processes
- Perform multi-channel time-lapse imaging to simultaneously monitor optogenetic actuator localization, cell membrane dynamics, and tissue-scale shape changes.
- For quantitative analysis of cell contractility, track myosin::GFP fluorescence intensity and apical cell area changes over time.
- Image at sufficient temporal resolution (typically 15-30 second intervals) to capture rapid cytoskeletal remodeling.
Data Analysis and Quantification
- Quantify changes in apical cell area and myosin intensity in light-stimulated versus control regions.
- Analyze tissue-scale deformation using particle image velocimetry or similar approaches.
- Correlate the magnitude and timing of optogenetic perturbations with subsequent changes in tissue morphology.
Instrumentation: Light-Sheet Microscopy for Optophysiology
The implementation of advanced optogenetics requires specialized microscopy platforms that combine precise light patterning with high-speed functional imaging. Light-sheet microscopy has emerged as the method of choice for these applications, particularly when studying small, translucent organisms such as larval zebrafish and Drosophila embryos [13]. This imaging modality provides unique advantages for all-optical physiology experiments, where both perturbation and readout are achieved through light.
Microscope Configurations and Performance Characteristics
Light-sheet microscopes can be implemented in various configurations, each with distinct advantages for specific experimental needs. The fundamental principle involves illuminating the sample with a thin sheet of light while collecting fluorescence signal at an orthogonal angle, enabling optical sectioning while minimizing phototoxicity [13].
Table 3: Light-Sheet Microscope Configurations for Optogenetic Applications
| Configuration | Key Features | Advantages | Ideal Applications |
|---|---|---|---|
| Selective Plane Illumination Microscopy (SPIM) | Two orthogonal objectives for illumination and detection [13] | Excellent optical sectioning, reduced photobleaching [13] | Long-term imaging of developing embryos |
| Digitally Scanned Light-Sheet Microscopy (DSLM) | Rapid scanning of a pencil beam to generate a "virtual" sheet [13] | Reduced light exposure, improved sectioning [13] | High-speed functional imaging |
| Multi-View Illumination | Multiple illumination and detection paths [13] | Improved resolution, robustness to sample opacity [13] | Imaging large or scattering samples |
| Swept Plane (Single Objective) | Single objective for both illumination and detection [13] | Simplified sample mounting, compatible with various samples [13] | High-throughput screening applications |
| Minimal-Complexity Systems | Add-on modules for standard microscopes [14] | Accessibility, rapid assembly, cost-effectiveness [14] | Standard laboratory environments |
Experimental Protocol: Minimal-Complexity Light-Sheet Imaging
Recent innovations have simplified light-sheet microscopy, making it more accessible for routine laboratory use. The following protocol describes the implementation of a minimal-complexity system for optogenetic applications [14]:
System Assembly and Alignment
- Replace the condenser of a standard inverted microscope with a light-sheet module.
- Align the light-sheet using screws typically used for Köhler illumination.
- Generate a static planar light-sheet using a cylindrical lens and a spherical symmetrical lens.
Sample Mounting and Preparation
- Mount samples in standard glass-bottom dishes.
- Minimize sample movement by embedding in agarose.
- Use long-working distance water-dipping objectives designed to operate with cover glasses.
Image Acquisition and Processing
- For volumetric scanning, move the sample through the light-sheet using a piezo stage.
- Acquire images at rates up to 10 ms/plane (100 fps) for calcium imaging applications.
- Process images to reduce typical light-sheet artifacts using specialized computational pipelines.
Data Analysis and Interpretation
The rich datasets generated by optogenetic experiments require specialized analytical approaches. For investigations of cell internalization during gastrulation, key analytical steps include:
- Quantification of Signaling Dynamics: Measure the spatial range and temporal dynamics of optogenetically activated signaling pathways using fluorescent biosensors.
- Cell Tracking and Trajectory Analysis: Monitor individual cell movements in response to patterned optogenetic stimulation using time-lapse imaging and segmentation algorithms.
- Gene Expression Analysis: Correlate the spatial patterns of optogenetic stimulation with subsequent domains of gene expression through in situ hybridization or live reporters.
- Force Inference and Mechanical Modeling: Infer cellular forces from observed deformations and integrate these data with mechanical models of tissue behavior.
The integration of optogenetics with light-sheet microscopy provides a powerful platform for all-optical physiology, enabling simultaneous readout and manipulation of biological systems with minimal phototoxicity [13]. This combination is particularly valuable for studying processes like gastrulation, where both precise spatial control and long-term observation are essential for understanding the underlying mechanisms.
Key Signaling Pathways and Mechanical Forces in Gastrulation
Gastrulation is a fundamental process in early embryonic development during which a single-layered blastula is reorganized into a multi-layered structure containing the primary germ layers (ectoderm, mesoderm, and endoderm). This transformation establishes the basic body plan of all complex animals. While biochemical signaling pathways have long been recognized as directors of this process, recent research has revealed that mechanical forces play an equally critical role in guiding gastrulation events. The emerging paradigm recognizes that successful gastrulation requires the precise integration of chemical signals with physical forces, where cells must be both chemically primed and physically prepared to execute morphogenetic programs [2] [3].
This application note explores the key signaling pathways and mechanical forces governing gastrulation, with particular emphasis on their relevance to light-controlled research methodologies. We focus specifically on the interplay between biochemical signaling and mechanical competence in regulating cell internalization events, providing detailed protocols for investigating these processes using advanced optogenetic tools. The insights gained from these approaches offer profound implications for understanding embryonic development, regenerative medicine, and fertility therapies [2] [15].
Key Signaling Pathways in Gastrulation
BMP4 Signaling and Axis Formation
The Bone Morphogenetic Protein 4 (BMP4) pathway serves as a primary regulator of symmetry breaking during gastrulation. BMP4 signaling helps establish the dorsal-ventral axis and triggers the initial events of germ layer specification. Recent studies using optogenetic tools have demonstrated that BMP4 activation alone is insufficient to drive complete gastrulation; it requires coordinated mechanical input to execute its full program [2] [3].
Mechanism of Action:
- BMP4 activation initiates transcription of target genes that promote extra-embryonic cell types
- Downstream effects are mediated through SMAD transcription factors
- Mechanical tension regulates BMP4 signaling efficacy through YAP/TAZ pathways
- BMP4 synergizes with WNT and Nodal signaling to establish positional information [2]
WNT and Nodal Signaling Cascades
WNT and Nodal signaling pathways function as critical downstream effectors in the gastrulation cascade. These pathways are fine-tuned by mechanical forces, creating a feedback loop that ensures precise spatial and temporal patterning of the developing embryo [2].
Interplay with Mechanical Forces:
- Mechanical tension via YAP1 fine-tunes WNT and Nodal signaling pathways
- These pathways instruct cells on their developmental fates and tissue specifications
- The mechanosensory protein YAP1 acts as a molecular brake on gastrulation, preventing premature transformation [2] [3]
Actomyosin-Based Contractility
Actomyosin networks generate the contractile forces necessary for cell shape changes and tissue remodeling during gastrulation. In C. elegans, apical accumulation and activation of non-muscle myosin II (NMY-2) is essential for endoderm internalization, demonstrating the conserved nature of this mechanism across species [10].
Table 1: Key Signaling Pathways in Gastrulation
| Pathway | Primary Components | Role in Gastrulation | Mechanical Regulation |
|---|---|---|---|
| BMP4 | BMP4 ligand, BMP receptors, SMAD transcription factors | Initiates symmetry breaking, dorsal-ventral patterning | Requires mechanical competence for full activation; regulated by YAP1 |
| WNT | WNT ligands, Frizzled receptors, β-catenin | Posterior patterning, mesoderm specification | Fine-tuned by mechanical tension via YAP1 |
| Nodal | Nodal ligand, Activin receptors, SMAD2/3 | Mesendoderm induction, left-right asymmetry | Mechanical forces modulate signaling amplitude |
| Actomyosin | Non-muscle myosin II, Actin filaments, Rho GTPases | Apical constriction, cell internalization | Responsive to tissue tension and geometrical constraints |
Mechanical Forces in Gastrulation
Tissue Geometry and Mechanical Confinement
The physical arrangement of cells and their mechanical environment play decisive roles in gastrulation progression. Studies using optogenetic activation of BMP4 in human embryonic stem cells have demonstrated that mechanical confinement is essential for proper gastrulation. When BMP4 was triggered in unconfined, low-tension environments, gastrulation never fully coalesced, yielding only extra-embryonic cell types while failing to generate mesoderm and endoderm layers [2] [3].
Key Findings:
- Cells at the edges of confined colonies experience sufficient tension to permit gastrulation
- Cells embedded in tension-inducing hydrogels form proper germ layers
- Tissue geometry influences force distribution and signaling efficacy
- Mechanical competence represents a permissive state for developmental progression [2]
YAP/TAZ Mechanosensing
The mechanosensory protein YAP1 (Yes-associated protein 1) serves as a critical integrator of mechanical and biochemical signals during gastrulation. Nuclear YAP1 levels respond to mechanical tension and act as a regulatory brake on gastrulation, preventing these transformations from occurring prematurely [2] [3].
Mechanosensing Mechanism:
- Mechanical tension regulates nuclear translocation of YAP1
- Nuclear YAP1 fine-tunes downstream biochemical signaling pathways
- YAP1 ensures gastrulation initiates only when mechanical conditions are permissive
- This mechanism prevents mistimed developmental transitions [2]
Rosette Formation and Actomyosin Dynamics
In C. elegans gastrulation, a distinct mode of cell internalization occurs through the formation of multicellular rosettes. This process involves coordinated actomyosin dynamics where internalizing cells display apical contractile flows while surrounding cells form centripetal extensions that converge to seal over the internalizing cells [10].
Characteristics of Rosette Formation:
- Exhibits modular structure and scalability
- Adapts to topological alterations through plastic organization
- Combines with coplanar cell division to enable volume rearrangement
- Represents a self-organizing system for piecemeal internalization [10]
Quantitative Data in Gastrulation Mechanics
Table 2: Quantitative Measurements of Mechanical Forces in Gastrulation
| Parameter | Experimental System | Measurement/Value | Biological Significance |
|---|---|---|---|
| Mechanical confinement effect | Human embryonic stem cells | 100% failure of mesendoderm formation in low-tension environments | Demonstrates absolute requirement for mechanical input in human gastrulation |
| Rosette formation efficiency | C. elegans embryo | 100% of embryos (n=13) showed rosette formation during endoderm internalization | Indicates highly conserved, robust mechanism for cell internalization |
| YAP1 nuclear localization | Human gastrula models | Serves as mechanical competence checkpoint | Prevents premature gastrulation until mechanical conditions are appropriate |
| Apical NMY-2 accumulation | C. elegans endoderm internalization | Required for apical constriction and internalization | Conserved mechanism for generating contractile forces |
| Tissue flow patterns | Zebrafish anterior neural plate | Opposing flows with vortices on either side of dorsal midline | Demonstrates large-scale force generation and coordination |
Optogenetic Control of Gastrulation: Experimental Protocols
Optogenetic Tool Development for BMP4 Activation
This protocol describes the implementation of a light-based synthetic embryo system for precise control of gastrulation initiation, enabling researchers to investigate the interplay between biochemical signaling and mechanical forces [2].
Materials Required:
- Human embryonic stem cells (hESCs)
- Optogenetic BMP4 activation construct (engineered hESCs with light-switchable BMP4)
- Custom light illumination system (specific wavelength, typically blue light)
- Micropatterned substrates or tension-inducing hydrogels
- Appropriate cell culture media and supplements
Procedure:
- Cell Preparation:
- Culture optogenetically engineered hESCs under standard conditions
- Passage cells onto micropatterned substrates or embed in tension-inducing hydrogels
- Allow cells to reach appropriate confluence (typically 70-80%)
Optogenetic Activation:
- Expose cells to specific wavelength of light to activate BMP4 signaling
- Control illumination parameters (duration, intensity, spatial pattern)
- For spatial control, use patterned illumination to activate specific regions of cell colonies
Mechanical Conditioning:
- Utilize confined colonies or tension-inducing hydrogels to provide mechanical input
- Vary substrate stiffness to test mechanical competence requirements
- Apply the mechanical context concurrently with optogenetic activation
Analysis and Validation:
- Monitor symmetry breaking and axis formation via time-lapse microscopy
- Assess germ layer specification using immunostaining for markers of ectoderm, mesoderm, and endoderm
- Evaluate YAP1 localization (nuclear vs. cytoplasmic) as indicator of mechanical signaling
- Quantify expression of downstream targets of BMP4, WNT, and Nodal signaling
Troubleshooting Tips:
- If gastrulation fails, verify mechanical context (confinement or hydrogel tension)
- Assess optogenetic construct functionality through control experiments
- Confirm appropriate cell density and colony size
- Validate mechanical competence through YAP1 localization assays [2] [3]
Quantifying Mechanical Forces in Avian Embryos
The avian embryo provides an excellent model for studying mechanical forces during gastrulation due to its accessibility for manipulation and imaging. This protocol focuses on measuring tissue-scale forces and cell behaviors in chick embryos [15].
Materials Required:
- Fertilized chick eggs (incubated to appropriate stage)
- Light sheet fluorescence microscope
- Microinjection equipment
- Fluorescent markers for cell membranes and nuclei
- Myosin inhibitors (e.g., blebbistatin) for perturbation studies
Procedure:
- Embryo Preparation:
- Window eggshell and visualize embryo under sterile conditions
- Inject fluorescent markers for cell membranes and nuclei
- Mount embryo for light sheet microscopy with minimal constraint
Live Imaging:
- Acquire time-lapse sequences of gastrulation movements
- Focus on regions of active intercalation and internalization
- Capture tissue-scale flows and cell-scale behaviors simultaneously
Force Inference and Quantification:
- Use particle image velocimetry (PIV) to map tissue flow fields
- Quantify cell intercalation behaviors and directions
- Measure apical constriction events in mesendoderm precursors
- Analyze myosin cable formation and dynamics
Mechanical Perturbation:
- Locally inhibit myosin activity using blebbistatin
- Physically constrain tissue movements using barriers
- Alter tissue tension through laser ablation experiments
Computational Modeling:
- Develop "digital twin" embryo models based on experimental measurements
- Simulate tissue flows incorporating both chemical and mechanical parameters
- Test predictions of models through further experiments [15]
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for Gastrulation Mechanics
| Reagent/Category | Specific Examples | Function/Application | Experimental Considerations |
|---|---|---|---|
| Optogenetic tools | Light-activatable BMP4, Channelrhodopsin-based systems | Precise spatiotemporal control of signaling pathways | Requires specialized illumination equipment; careful calibration of light parameters |
| Mechanosensing reporters | YAP/TAZ localization biosensors, FRET-based tension sensors | Visualizing mechanical force transmission and cellular response | Critical for establishing mechanical competence status |
| Cytoskeletal modulators | Blebbistatin (myosin inhibitor), Y-27632 (ROCK inhibitor) | Perturbing actomyosin-based contractility | Dose-dependent effects; potential compensatory mechanisms |
| Synthetic substrates | Micropatterned surfaces, tunable hydrogels | Controlling tissue geometry and mechanical environment | Stiffness and pattern dimensions must be optimized for specific cell types |
| Live imaging reagents | Membrane dyes, nuclear labels, vital fluorescent proteins | Tracking cell behaviors and tissue dynamics in real-time | Phototoxicity concerns; balance between signal intensity and cell health |
| Mathematical modeling platforms | Vertex models, cellular Potts models, continuum mechanics frameworks | Simulating tissue morphogenesis and predicting emergent behaviors | Requires integration with experimental data for validation |
| Cyclopropyl 2,6-dimethylphenyl ketone | Cyclopropyl 2,6-Dimethylphenyl Ketone|CAS 870002-28-1 | Bench Chemicals | |
| Potassium 4-bromobenzenesulfonate | Potassium 4-bromobenzenesulfonate, CAS:66788-58-7, MF:C6H4BrKO3S, MW:275.16 g/mol | Chemical Reagent | Bench Chemicals |
Signaling and Mechanical Pathway Diagrams
The integration of optogenetic tools with mechanical manipulation has revolutionized our understanding of gastrulation, revealing the profound interdependence between biochemical signaling and physical forces. The methodologies outlined in this application note provide researchers with robust protocols for investigating the mechanochemical control of cell internalization during this critical developmental window.
Future research directions will likely focus on identifying the potential existence of a mechanical organizer—a force-based counterpart to classical signaling centers that shape the early embryo. The concept of mechanical competence provides a framework for understanding how embryos satisfy specific physical conditions to progress through developmental milestones. These advances not only illuminate fundamental biology but also hold promise for regenerative medicine and fertility therapies, potentially leading to improved stem-cell therapies that activate on demand and better understanding of early pregnancy failures [2] [3].
The tools and approaches described here—from optogenetic control to quantitative force measurements—establish a foundation for continued exploration of how mechanical and chemical information integrate to build complex organisms from simple embryonic structures.
Optogenetics is fundamentally reshaping biological research by enabling unprecedented precise, spatiotemporal control over cellular processes. This technique, which uses light to manipulate the activity of genetically targeted cells, has evolved from a tool for observing neural circuits to a powerful platform for active intervention in complex biological systems. By shifting the research paradigm from passive observation to directed perturbation, optogenetics allows scientists to not just watch biological processes unfold, but to actively test hypotheses about causal mechanisms by manipulating specific pathways with cellular and temporal precision. This paradigm shift is particularly transformative in developmental biology, where it enables researchers to dissect the intricate signaling networks and mechanical forces that orchestrate embryogenesis. Within this context, the application of optogenetics to study gastrulation—a pivotal event in early embryonic development where massive cell rearrangements establish the basic body plan—represents a frontier where this technology is yielding profound new insights into how cells internalize to form the three germ layers.
Application Notes: Optogenetic Control of Morphogenetic Processes
Decoding Symmetry Breaking in Human Gastrulation Models
The breaking of radial symmetry during gastrulation represents one of the most fundamental transformations in embryonic development. Recent research has leveraged optogenetics to dissect the intricate crosstalk between biochemical signaling and tissue mechanics that regulates this process. In a landmark study, scientists established a light-inducible BMP4 expression system in human embryonic stem cells (hESCs) to investigate how spatially localized morphogen signaling initiates symmetry breaking and germ layer specification [16].
This optogenetic approach revealed several key insights:
- Mechanical Regulation: Tissue mechanics at the epiblast border directly regulate BMP4 signaling competency, creating a feedback loop that patterns fate acquisition
- Signaling Hierarchy: Light-controlled BMP4 activation sequentially induces WNT and YAP signaling, establishing a hierarchical signaling cascade that guides cell fate decisions
- Geometric Control: The combination of optogenetic stimulation with micropatterned confinement enabled quantitative analysis of how biochemical and mechanical signals integrate to drive self-organization
The experimental paradigm combined optogenetic-programmable hESCs with mathematical modeling to formally demonstrate that mechanical signals are crucial regulators of the BMP4 signaling cascade during in vitro gastrulation [16]. This work exemplifies how optogenetics moves beyond correlation to establish causality in developmental processes.
Quantitative Control of Epithelial Morphogenesis
Beyond mammalian systems, optogenetics has enabled equally profound insights into the biophysical principles of epithelial tissue folding in Drosophila. Researchers recently developed endogenous OptoRhoGEFs by using CRISPR/Cas9 to tag Drosophila RhoGEF2 and Cysts/Dp114RhoGEF with components of the iLID/SspB optogenetic heterodimer [17]. This innovative approach permitted:
- Dose-Dependent Control: Quantitative manipulation of RhoGEF recruitment revealed a dose-dependent relationship between RhoGEF activity and epithelial furrow depth, demonstrating how embryos can shape tissues into specific morphologies by tuning signaling intensity
- Subcellular Precision: Two-photon activation protocols enabled z-specificity of 4.2 ± 0.3 µm, allowing precise spatial control over contractility at the subcellular level
- Viable Development: Unlike transgenic overexpression tools that often compromise embryonic viability, endogenously tagged OptoRhoGEFs maintained normal development while enabling optogenetic perturbation
This system uncovered that at gastrulation onset, furrows formed by cell lateral contraction are oriented and size-constrained by basal actomyosin, revealing how mechanical context shapes the outcome of contractile processes [17].
Table 1: Quantitative Parameters for Optogenetic Control of Morphogenesis
| Biological Process | Optogenetic Tool | Control Precision | Key Quantitative Finding | Reference |
|---|---|---|---|---|
| Human gastrula model symmetry breaking | Light-inducible BMP4 | Spatial patterning on micropatterns | Mechanical signals regulate BMP4 competency at epiblast border | [16] |
| Drosophila epithelial furrowing | Endogenous OptoRhoGEFs (RhoGEF2, Cysts) | Z-specificity: 4.2 ± 0.3 µm | Furrow depth shows dose-dependence on RhoGEF recruitment | [17] |
| Cortical astrocyte calcium signaling | ChR2(C128S) in MlC1+ astrocytes | Duty cycle optimization (20-95% of T=100s) | 20% paradigm elicits robust calcium responses across stimulations | [18] |
Calcium Signaling in Feather Morphogenesis
The power of optogenetics extends to appendage development, as demonstrated by research on chicken embryos investigating calcium's role in feather growth. Using the Opto-CRAC photogenetic tool to control calcium influx, researchers discovered that feather mesenchymal cells display synchronous calcium oscillations that correlate with elongation rate [19]. This application highlights how optogenetic intervention can:
- Manipulate Specific Pathways: Light activation of Opto-CRAC induced calcium influx and promoted feather growth, while inhibition reduced elongation rates
- Establish Causality: Demonstrated that calcium oscillations are not merely correlated with but actively drive morphogenetic processes
- Interface with Native Development: Combined with the accessibility of chicken embryos, enabled site-specific control of cell activity during ongoing development
Experimental Protocols
Protocol for Light-Inducible BMP4 Signaling in hESC Gastruloids
This protocol enables precise spatiotemporal control of BMP signaling to investigate symmetry breaking in human gastrula models [16]:
Materials and Reagents:
- RUES2 hESCs (NIH hESC-09-0013)
- piggyBac vector with human BMP4 downstream of loxP-flanked stop cassette
- Doxycycline (for light sensitivity induction)
- Micropatterned substrates (for geometrical confinement)
- Blue light source (470 nm, with precise spatial control)
Procedure:
- Cell Preparation: Culture hESCs in defined pluripotency-maintaining conditions
- Genetic Modification: Insert the light-inducible BMP4 construct via piggyBac transposition
- Sensitization: Treat with doxycycline to confer light sensitivity (induces Cre recombinase expression)
- Patterning: Plate cells on micropatterned substrates to establish geometrical confinement
- Optogenetic Stimulation: Apply spatially defined blue light (470 nm) to induce BMP4 expression in specific regions
- Fixation and Analysis: Fix cells at appropriate timepoints for immunostaining of germ layer markers
Key Parameters:
- Light intensity: 10-50 µW/mm²
- Stimulation duration: 5-60 minutes, depending on desired signaling strength
- Optimal timing: Apply stimulation during early stages of micropattern culture (0-24h)
Troubleshooting:
- If spontaneous differentiation occurs: Optimize doxycycline concentration and exposure time
- If patterning is inconsistent: Verify micropattern quality and cell seeding density
- If light response is weak: Verify construct integration and light source calibration
Protocol for Endogenous OptoRhoGEFs in Drosophila Embryos
This protocol describes the use of CRISPR-generated OptoRhoGEFs for quantitative manipulation of epithelial morphogenesis [17]:
Materials and Reagents:
- Drosophila strains with endogenously tagged RhoGEF2-tgRFPt-SspB or SspB-tgRFPt-Cysts
- UASp>mCherry-iLID-CaaX (or similar iLID constructs)
- Two-photon microscope (920 nm for activation, 1040 nm for imaging)
- Embryo collection cages and apple juice agar plates
Procedure:
- Fly Crosses: Cross virgin females from OptoRhoGEF lines to males carrying maternal GAL4 driver and UASp>iLID-CaaX
- Embryo Collection: Collect 0-3 hour old embryos and dechorionate manually
- Mounting: Align embryos on glass-bottom dishes and cover with halocarbon oil
- Imaging and Activation: Use two-photon microscopy at 1040 nm for imaging and 920 nm for optogenetic activation
- Patterned Illumination: Apply light in user-defined patterns (e.g., lines, circles) to recruit RhoGEFs to specific membrane domains
- Quantitative Analysis: Measure furrow depth, myosin intensity, and cell shape changes
Key Parameters:
- Laser power: 5-20 mW (two-photon)
- Pixel dwell time: 2-10 µs
- Activation patterns: 1-5 µm lines or circles, 5-30 second exposure
- Optimal developmental stage: Cellularization through gastrulation
Troubleshooting:
- If recruitment is inefficient: Increase iLID expression levels or laser power
- If embryos are unhealthy: Reduce laser power and optimize collection timing
- If specificity is poor: Verify two-photon calibration and use z-stack activation
Table 2: Optical Stimulation Parameters for Different Experimental Systems
| System | Light Wavelength | Intensity/Duty Cycle | Temporal Pattern | Optimal Readouts |
|---|---|---|---|---|
| hESC gastruloids [16] | 470 nm (blue) | 10-50 µW/mm² | Single or multiple pulses, 5-60 min | pSMAD1/5, BRA, GATA6, Sox17 |
| Drosophila embryos (OptoRhoGEFs) [17] | 920 nm (two-photon) | 5-20 mW | 5-30 sec patterned illumination | Myosin, cell shape, furrow depth |
| Cortical astrocytes [18] | 470 nm (blue) | 20% duty cycle (20s/100s) | Periodic stimulation (T=100s) | Calcium (Rhod-2 AM), blood flow |
| Chicken embryo feather morphogenesis [19] | Blue light (ChR2) | Cell-type specific | Rhythmic or sustained | Calcium oscillation, elongation rate |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for Optogenetic Gastrulation Studies
| Reagent / Tool Name | Type | Function in Experiment | Example Application |
|---|---|---|---|
| Channelrhodopsin-2 (ChR2) | Microbial opsin | Cation channel that depolarizes cells upon blue light exposure | Neural activity control in chicken embryos [19] |
| Opto-CRAC | Engineered optogenetic tool | Controls calcium influx through CRAC channels | Feather morphogenesis in chicken embryos [19] |
| Light-inducible BMP4 | Optogenetic gene expression system | Enables spatial control of BMP4 morphogen signaling | Symmetry breaking in human gastruloids [16] |
| Endogenous OptoRhoGEFs (RhoGEF2, Cysts) | CRISPR-tagged endogenous proteins | Provides quantitative control over Rho signaling | Epithelial furrowing in Drosophila [17] |
| iLID/SspB system | Optogenetic heterodimerizer | Enlight-induced protein recruitment to membrane | Subcellular control of contractility [17] |
| MlC1-ChR2(C128S)-EYFP mouse | Transgenic animal model | Enables astrocyte-specific optogenetic stimulation | Calcium signaling in cortical astrocytes [18] |
| Pisces (Photo-inducible single-cell labeling) | Multimodal marking tool | Links neuronal morphology, activity, and molecular information | Neural circuit mapping in zebrafish [20] |
| 4,6-Dihydroxynaphthalene-2-sulphonic acid | 4,6-Dihydroxynaphthalene-2-sulphonic acid, CAS:6357-93-3, MF:C10H8O5S, MW:240.23 g/mol | Chemical Reagent | Bench Chemicals |
| 2,4-Dichloroquinoline-3-carbonitrile | 2,4-Dichloroquinoline-3-carbonitrile, CAS:69875-54-3, MF:C10H4Cl2N2, MW:223.05 g/mol | Chemical Reagent | Bench Chemicals |
Signaling Pathways and Experimental Workflows
Optogenetic Control of Gastrulation Signaling Networks This diagram illustrates the core signaling pathways manipulated by optogenetic tools during gastrulation. The framework shows how light input simultaneously regulates biochemical signaling (green) through controlled morphogen expression and mechanical signaling (blue) through precise Rho pathway activation, with feedback mechanisms integrating these pathways to drive gastrulation outcomes.
Experimental Workflow for Optogenetic Gastrulation Studies This workflow outlines the key stages in optogenetic experiments, from sample preparation through intervention to analysis. The process begins with genetic modification of cells or embryos (yellow), proceeds through precisely controlled light stimulation (yellow), incorporates quantitative analysis (blue), and culminates in mechanistic insights (red) that advance our understanding of developmental principles.
Optogenetics has fundamentally transformed our approach to studying embryonic development by providing a precision intervention toolkit that moves beyond correlation to establish causality. The applications detailed in this article—from controlling symmetry breaking in human gastruloids to quantitatively manipulating epithelial furrowing in Drosophila—demonstrate how this technology enables researchers to actively probe the mechanical and biochemical principles of morphogenesis. As the field advances, several exciting frontiers are emerging: the integration of bioluminescent optogenetics that eliminates the need for external light sources [21], the development of multimodal single-cell mapping tools like Pisces that bridge cellular structure, function, and molecular identity [20], and the creation of more physiological model systems that better recapitulate the mechanical context of native development. These advances, combined with increasingly sophisticated mathematical modeling approaches, promise to further unravel the exquisite precision of embryonic patterning while providing new engineering principles for tissue design and regenerative medicine applications. As optogenetic methodologies continue to evolve, they will undoubtedly uncover deeper insights into how collective cell behaviors emerge from individually programmed instructions—a central mystery not just of development, but of life itself.
The Optogenetic Toolkit: Methods for Light-Control of Cell Internalization and Fate
The precise control of cellular processes with light, known as optogenetics, has revolutionized biological research by enabling manipulation of protein activity with exceptional spatiotemporal precision. For researchers investigating complex morphological events such as gastrulation—a critical developmental stage where a simple sheet of cells reorganizes into the foundational blueprint of the body—these tools offer unprecedented power to dissect causal relationships. The success of such investigations hinges on selecting the appropriate light-sensitive protein. This application note provides a detailed comparison of three principal optogenetic systems—LOV domains, Cryptochrome 2 (CRY2), and Phytochromes—and offers specific protocols for their use in studying cell internalization during gastrulation.
Comparison of Major Optogenetic Systems
The table below summarizes the core characteristics of the three major classes of photosensitive proteins, providing a basis for selection.
Table 1: Key Characteristics of Major Photosensitive Proteins
| Feature | LOV Domains (e.g., AsLOV2, VfAu1-LOV) | Cryptochrome 2 (CRY2) | Phytochromes (e.g., AtPhyB, Cph1) |
|---|---|---|---|
| Activation Light | Blue light (~450 nm) [22] [23] | Blue light (~430-490 nm) [24] [25] | Red light (~650 nm) [26] [23] |
| Reversion/Deactivation | Spontaneous in darkness (seconds-minutes) [22] | Spontaneous in darkness (half-life ~5.5 min) or with green light [25] | Far-red light (>750 nm) [26] |
| Primary Mechanism | Conformational change (Jα helix undocking) [22] | Homo-oligomerization & hetero-dimerization (with CIB1) [24] | Hetero-dimerization (with PIF proteins) [26] [27] |
| Exogenous Cofactor | No (binds FMN/FAD) [22] | No (binds flavin) [24] [25] | Yes (e.g., Phycocyanobilin - PCB) [26] |
| Key Engineering Variants | iLID, pmLID [23] | CRY2olig (E490G), CRY2high, CRY2low [24] [23] | Optimized PhyB-PIF6 pair, minimal PIF fragments [26] [27] |
| Typical Activation Kinetics | Seconds [22] | Subseconds to seconds [25] | ~1.3 seconds [26] |
| Typical Deactivation Kinetics | Seconds to minutes [22] | Minutes (in darkness) [25] | ~4 seconds [26] |
| 5-Undecyl-1h-1,2,4-triazol-3-amine | 5-Undecyl-1h-1,2,4-triazol-3-amine, CAS:92168-88-2, MF:C13H26N4, MW:238.37 g/mol | Chemical Reagent | Bench Chemicals |
| 5-(Thiophen-2-yl)nicotinaldehyde | 5-(Thiophen-2-yl)nicotinaldehyde|CAS 342601-29-0 | 5-(Thiophen-2-yl)nicotinaldehyde (CAS 342601-29-0) is a high-purity building block for antimalarial and kinase inhibitor research. This product is for Research Use Only (RUO). Not for human or veterinary use. | Bench Chemicals |
The following diagram illustrates the core operational mechanisms of these three protein systems.
Quantitative Performance Data
For experimental design, understanding the quantitative performance of engineered variants is critical. The following tables consolidate key metrics.
Table 2: Performance Metrics of Engineered CRY2 Variants
| CRY2 Variant | Key Mutation/Feature | Oligomerization Propensity | Primary Application |
|---|---|---|---|
| CRY2wt | Wild-type PHR domain | Moderate (membrane-enhanced) [25] | Baseline studies |
| CRY2olig | E490G | Enhanced [24] [23] | Robust clustering and activation |
| CRY2high | Engineered C-terminal positive charges | Drastically enhanced [24] | Applications requiring strong oligomerization |
| CRY2low | Engineered C-terminal negative charges | Suppressed [24] | CRY2-CIB1 heterodimerization with minimal unintended clustering |
Table 3: Performance Metrics of Phytochrome-PIF Interactions
| Interaction Pair | Dissociation Constant (Kd) | Activation Kinetics (Ï„) | Reversion Kinetics (Ï„) | Notes |
|---|---|---|---|---|
| AtPhyB PCM - P6.100 | ~10 nM (Pfr state) [27] | N/A | N/A | Very low Pr-state affinity [27] |
| Optimized PhyB-PIF6 | ~20-100 nM (in vivo est.) [26] | 1.3 ± 0.1 s [26] | 4 ± 1 s [26] | Robust, reversible, >100 cycles [26] |
| Minimal PIF Variant (25aa) | Retained binding [27] | N/A | N/A | Reduced basal activity, enhanced light response [27] |
Experimental Protocols for Gastrulation Research
The following protocols are adapted from foundational studies, with a focus on investigating the interplay of biochemical signaling and mechanical forces during gastrulation, a process pivotal to axis formation in early development [2].
Protocol 1: Optogenetic Control of BMP4 Signaling in Synthetic Embryos
This protocol is designed to replicate the critical gastrulation signaling event in a controlled, synthetic embryo model [2].
- Objective: To spatiotemporally activate BMP4 signaling in human embryonic stem cells (hESCs) to study its role in axis formation and cell fate specification, contingent on mechanical force.
- Key Reagents:
- Optogenetic hESC Line: hESCs engineered to express a light-inducible BMP4 gene switch (e.g., CRISPR-engineered locus with blue light-sensitive transcription factor).
- Microfabricated Substrates: Micropatterned chips or tension-inducing hydrogels to control colony geometry and mechanical stress [2].
- PCB Chromophore: For PhyB-PIF systems, supplement culture medium with 5µM PCB for 30+ minutes prior to experimentation [26].
- Procedure:
- Cell Seeding and Confinement: Seed optogenetic hESCs onto micropatterned substrates or within tension-inducing hydrogels to create defined mechanical conditions [2].
- Chromophore Incubation (if using Phytochrome): Incubate cells with PCB-containing medium to ensure proper chromophore incorporation [26].
- Light Stimulation Pattern:
- Use a digital micromirror device (DMD) to project precise patterns of activating light (blue for CRY/LOV, red for PhyB) onto the cell colonies.
- Target the periphery of confined colonies, as this is where mechanical tension facilitates successful gastrulation-like transformations [2].
- For sustained activation, use pulsed illumination (e.g., 5-second pulse every 5 minutes) [23].
- Live Imaging and Analysis:
- Monitor the formation of germ layers (mesoderm, endoderm) via live-cell imaging of specific fluorescent reporters (e.g., BRA::YFP for mesoderm).
- Fix cells at specific timepoints and perform immunofluorescence for key transcription factors (e.g., BRACHYURY, SOX17) to quantify cell fate specification.
- Assess the nuclear localization of YAP1 as a readout of mechanical force integration [2].
Protocol 2: Light-Actuated Membrane Recruitment to Modulate Signaling
This generalizable protocol uses light to recruit proteins to the membrane, a common mechanism for activating signaling pathways [26] [23].
- Objective: To activate Rho-family GTPase signaling pathways (e.g., Rac1, Cdc42) controlling cytoskeleton remodeling and cell morphology by optically recruiting their GEFs.
- Key Reagents:
- Procedure:
- Cell Transfection: Co-transfect NIH3T3 or HEK293T cells with plasmids encoding the membrane "Bait" and cytosolic "Prey" constructs.
- Chromophore Incubation: For phytochrome systems, incubate with PCB.
- Global Recruitment Test: Expose the entire cell culture dish to activating light to validate system functionality. Observe for global morphological changes (e.g., lamellipodia formation) within 20-30 minutes [26].
- Spatiotemporal Recruitment:
- Use a confocal microscope with a laser scanning module or DMD for patterned illumination.
- To create a sharp spot of activation, bathe the sample in continuous "inactivating" light (e.g., far-red for PhyB) while simultaneously delivering pulses of "activating" light (e.g., red for PhyB) to a focused point. This traps the recruited protein at the target location [26].
- Monitor actin cytoskeleton dynamics in real-time using a fluorescent marker like LifeAct.
Signaling Pathways in Development and Experimentation
The diagram below outlines a core signaling pathway relevant to gastrulation studies and how optogenetic tools can be used to probe it, based on recent findings [2].
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Reagent Solutions for Optogenetic Gastrulation Studies
| Reagent / Tool | Function | Example Application in Gastrulation |
|---|---|---|
| CRY2-CIB1 Pair | Blue-light-induced hetero-dimerization [24] [25] | Recruiting GEFs to the membrane to activate small GTPases and study cytoskeletal remodeling [26]. |
| CRY2olig / CRY2high | Enhanced blue-light-induced homo-oligomerization [24] [23] | Clustering and activating receptor tyrosine kinases (e.g., FGFR, EphB2) to mimic ligand-induced signaling [23]. |
| Optimized PhyB-PIF Pair | Red-light-induced, far-red-reversible dimerization [26] [27] | High-precision, reversible membrane recruitment of signaling domains; used in synthetic embryo models for axial patterning [26] [2]. |
| LOV-based OptoNBs | Light-switchable nanobodies binding endogenous proteins [28] | Inhibiting or activating untagged endogenous proteins like GFP-tagged transcription factors without genetic modification. |
| LOV2-Jα Peptide Fusions | Blue-light-uncaging of functional peptides (NLS, degrons, inhibitors) [22] | Controlling nuclear import of transcription factors or inducing degradation of key signaling proteins. |
| PCB Chromophore | Essential cofactor for phytochrome function [26] | Reconstituting PhyB activity in mammalian cells; typically used at 5µM [26]. |
| Micropatterned Substrates | Controlling cell colony geometry and mechanical tension [2] | Providing the mechanical context necessary for BMP4 to initiate successful gastrulation in synthetic embryos [2]. |
| Digital Micromirror Device (DMD) | Projecting user-defined patterns of light with micron-scale resolution [26] | "Painting" activation signals onto cell membranes or tissues to create custom morphogen gradients. |
| 2-Chlorobenzophenone ethylene ketal | 2-Chlorobenzophenone ethylene ketal, CAS:760192-90-3, MF:C15H13ClO2, MW:260.71 g/mol | Chemical Reagent |
| 2-Butoxy-N-(2-methoxybenzyl)aniline | 2-Butoxy-N-(2-methoxybenzyl)aniline | 2-Butoxy-N-(2-methoxybenzyl)aniline is a high-quality chemical reagent for research use only (RUO). Not for human or veterinary diagnostic or therapeutic use. |
The choice between LOV domains, CRY2, and Phytochromes is not one of superiority but of strategic application. For the fastest, blue-light-controlled conformational changes, LOV domains are ideal. CRY2 offers a powerful and facile system for inducing oligomerization and clustering with blue light, though its dual nature requires careful consideration. For the highest spatiotemporal precision, reversible control, and minimal background activity, the Phytochrome-PIF system is unparalleled, albeit with the added complexity of a cofactor. By leveraging the protocols, data, and reagents detailed herein, researchers can design robust experiments to deconstruct the intricate interplay of biochemical and mechanical signals that orchestrate gastrulation and other fundamental developmental processes.
The precise control of gene expression with high spatiotemporal resolution has become an essential capability for probing complex biological processes, particularly during embryonic development. Light-inducible systems represent a groundbreaking technological advancement that enables researchers to manipulate genetic activity and signaling pathways with unprecedented precision. In the context of studying cell internalization during gastrulation—a critical phase in early embryonic development where the body's fundamental axes are established—these optogenetic tools provide a powerful means to dissect the underlying molecular and mechanical principles without the limitations of traditional chemical inducers or genetic perturbations [29] [6].
The fundamental advantage of optogenetic control lies in its non-invasive nature and the ability to deliver precise perturbations in both space and time. During gastrulation, a flat sheet of cells undergoes dramatic transformation, folding into distinct layers and axes that serve as a blueprint for subsequent tissue development [29]. This process involves intricate patterns of signaling activity and mechanical forces that have been difficult to study with conventional methods. Light-inducible systems now offer researchers the capability to manipulate these patterns with cellular and even subcellular resolution, opening new avenues for investigating how embryonic cells decode positional information to make appropriate fate decisions [6].
This application note provides a comprehensive overview of current light-inducible technologies, detailed protocols for their implementation, and specific methodologies for applying these systems to study cell internalization during gastrulation. By framing this information within the context of developmental biology research, we aim to equip scientists with the practical knowledge needed to leverage these powerful tools in their investigations of embryonic patterning and morphogenesis.
Optogenetic Systems for Gene Expression Control
Key Design Principles and Mechanisms
Optogenetic systems for controlling gene expression typically function by coupling light-sensitive protein domains to transcriptional activators or repressors, or to key signaling components that can influence downstream genetic networks. The core principle involves using specific wavelengths of light to induce conformational changes in these photosensitive domains, leading to the activation or recruitment of effector modules that modulate gene expression [30]. Most systems are designed with several key characteristics in mind: high dynamic range (significant difference between dark and light states), low background activity in the absence of light, rapid response kinetics, reversibility, and compatibility with biological systems without causing phototoxicity.
These systems generally fall into two main categories: those based on oligomerization/dimerization of photoreceptor domains and those utilizing conformational changes within single photoreceptor proteins. Oligomerization-based systems, such as those employing the Cry2/CIB1 or iLID/SspB pairs, bring together separate DNA-binding and activation domains upon light illumination [6] [17]. Conformational change systems, such as those utilizing LOV domains or phytochromes, typically release autoinhibitory constraints or expose functional domains when exposed to light [31].
Advanced Systems for Research Applications
Recent advancements have yielded increasingly sophisticated optogenetic tools with improved performance characteristics. The DEL-VPR system represents a particularly potent approach for mammalian cells, fusing the blue light-activated EL222 receptor from Erythrobacter litoralis to three transcriptional activator domains (VP64, p65, and Rta) in tandem. This configuration achieves remarkable induction levels of up to 570-fold upon blue light stimulation, reaching expression levels comparable to strong constitutive promoters [31]. This system has been successfully implemented for light-induced expression of complex therapeutic proteins, including monoclonal and bispecific antibodies, with reduced byproduct formation and increased functional protein yields [31].
For neuronal applications, the Pisces (Photo-inducible single-cell labeling system) enables rapid, complete, and stable labeling of arbitrary neurons in intact animals such as larval zebrafish. This tool utilizes a nuclear chimeric protein containing a photo-cleavable protein (PhoCl), a photoconvertible fluorescent protein (mMaple), and a balanced combination of nuclear localization signal (NLS) and nuclear export signal (NES) [32]. Upon violet light activation, the cleaved and photoconverted mMaple is actively transported throughout the neuron by NES, enabling comprehensive tracing of complex neuronal morphologies. This system is particularly valuable for linking individual neurons' morphological characteristics with their functional properties and gene expression profiles [32].
The optoNodal2 system represents a specialized tool for developmental biology, specifically designed for controlling Nodal signaling patterns in zebrafish embryos. This improved version eliminates dark activity and improves response kinetics while maintaining a strong dynamic range [6]. By adapting an ultra-widefield microscopy platform for parallel light patterning in up to 36 embryos simultaneously, researchers can achieve precise spatial control over Nodal signaling activity and downstream gene expression, making it particularly valuable for studying gastrulation processes [6].
For manipulating morphogenetic processes, endogenous OptoRhoGEFs provide precise control over Rho signaling, which directs epithelial morphogenesis during development. By using CRISPR/Cas9 to tag endogenous Drosophila RhoGEF2 and Cysts/Dp114RhoGEF with components of the iLID/SspB optogenetic heterodimer, researchers can achieve light-dependent control over native protein activities without the variability associated with transgenic overexpression [17]. This approach has revealed dose-dependent relationships between RhoGEF recruitment and epithelial furrowing, providing insights into how embryos shape tissues into specific morphologies [17].
A novel approach to optical control of gene expression involves DNA G-quadruplex (G4) targeting reversible photoswitches. This method utilizes azobenzene-derived molecules that selectively bind to G-quadruplex DNA structures—four-stranded configurations formed by guanine-rich sequences in promoter regions—and modulate their conformation in response to specific light wavelengths [33]. The system functions as a reversible genetic "dimmer switch," allowing fine-tuning of gene expression with high temporal resolution by stabilizing or relaxing G4 structures to impede or permit transcription factor binding [33].
Table 1: Comparison of Advanced Light-Inducible Systems
| System Name | Light Sensitivity | Mechanism of Action | Dynamic Range/Performance | Primary Applications |
|---|---|---|---|---|
| DEL-VPR | Blue light (≈450 nm) | Light-oxygen-voltage (LOV) domain fused to transcriptional activator domains | Up to 570-fold induction; reaches levels of strong constitutive promoters | Bioproduction of complex proteins; basic research in mammalian cells |
| Pisces | Violet light (405 nm) | Nuclear PhoCl-mMaple fusion with NLS/NES balance; photo-cleavage enables cytosolic distribution | Rapid neurite labeling (1.02 ± 0.06 μm/s); compatible with live imaging | Single-neuron morphology tracing; multimodal neuronal profiling |
| optoNodal2 | Blue light (≈488 nm) | Cry2/CIB1N heterodimerization of Nodal receptors with cytosolic sequestration | Eliminates dark activity; improved kinetics; high dynamic range | Patterning Nodal signaling in zebrafish embryos; gastrulation studies |
| Endogenous OptoRhoGEFs | Blue light (488 nm) | iLID/SspB heterodimerization targeting endogenous RhoGEFs to membrane | Highly consistent expression (10-15% fluctuation); improved embryonic viability | Quantitative epithelial morphogenesis; tissue furrowing mechanisms |
| G4 Photoswitch | Dual-wavelength visible light | Azobenzene isomerization stabilizes/relaxes G-quadruplex DNA structures | Reversible control; minimal photodamage; deep tissue penetration possible | Dynamic gene regulation; potential therapeutic applications |
Application Notes for Gastrulation Research
Controlling Cell Internalization During Gastrulation
Gastrulation represents a pivotal stage in early embryonic development, characterized by extensive cell rearrangements and tissue remodeling that establish the fundamental body plan. During this process, coordinated cell internalization movements transform a simple epithelial sheet into the three primary germ layers (ectoderm, mesoderm, and endoderm) that will give rise to all tissues and organs [29]. The mechanical forces driving these morphological changes are precisely regulated by spatial patterns of signaling activity, with the Nodal pathway playing a particularly important role in mesendodermal patterning and cell internalization behaviors [6].
Light-inducible systems offer unprecedented opportunities to investigate the mechanisms governing gastrulation by enabling precise manipulation of key signaling pathways with high spatiotemporal control. The optoNodal2 system, for example, allows researchers to create designer Nodal signaling patterns in live zebrafish embryos, directly testing how specific spatiotemporal profiles of morphogen activity instruct cell fate decisions and internalization behaviors [6]. Similarly, endogenous OptoRhoGEF tools enable quantitative manipulation of the contractile forces that drive epithelial bending and furrowing during gastrulation stages [17].
Experimental Design Considerations
When designing experiments to study cell internalization during gastrulation using light-inducible systems, several critical factors must be considered:
Temporal Precision: Gastrulation involves rapidly evolving patterns of gene expression and cell behaviors. Optogenetic systems with fast activation and deactivation kinetics (such as optoNodal2) are essential for delivering precisely timed perturbations that match the natural tempo of developmental processes [6].
Spatial Patterning: The ability to create specific spatial patterns of signaling activity is crucial for probing how positional information is decoded during gastrulation. Widefield illumination systems capable of projecting complex patterns onto multiple embryos simultaneously enable high-throughput screening of different signaling geometries [6].
Physiological Relevance: Systems that operate at endogenous expression levels (such as endogenously tagged OptoRhoGEFs) minimize artifacts associated with overexpression and provide more physiologically relevant readouts of developmental mechanisms [17].
Multimodal Integration: Combining optogenetic perturbations with live imaging of cell behaviors and eventual molecular profiling provides comprehensive insights into the relationship between signaling patterns, cell mechanics, and fate decisions [32].
Detailed Experimental Protocols
Protocol 1: Optogenetic Control of Nodal Signaling in Zebrafish Embryos
This protocol describes the procedure for using the optoNodal2 system to control Nodal signaling patterns in zebrafish embryos during gastrulation stages, enabling precise manipulation of cell internalization processes.
Materials and Reagents:
- Zebrafish embryos with optoNodal2 transgenes (type I receptor acvr1b and type II receptor acvr2b fused to Cry2/CIB1N)
- Blue LED illumination system (470-490 nm) with spatial patterning capability
- Widefield fluorescence microscope with environmental chamber
- Embryo medium (E3)
- Agarose-coated imaging dishes
- RNA in situ hybridization reagents for Nodal target genes (e.g., gsc, ntl, sox32)
Procedure:
- Embryo Preparation and Mounting:
- Collect zebrafish embryos at the 1-4 cell stage and maintain in E3 medium at 28.5°C.
- At sphere stage (4 hours post-fertilization), dechorionate embryos manually with fine forceps.
- Arrange 20-36 embryos in an agarose-coated imaging dish with animal poles oriented consistently.
- Add sufficient E3 medium to submerge embryos while preventing floating.
Light Patterning Setup:
- Program desired illumination patterns using the spatial light modulator software.
- Configure pulse parameters (typically 5-30 μW/mm² intensity with 30 sec on/90 sec off cycles).
- Align embryos with the projected pattern using low-intensity brightfield illumination.
Optogenetic Activation:
- Initiate light patterning at 50% epiboly (5.3 hpf) to coincide with endogenous Nodal signaling onset.
- Continue patterned illumination through shield stage (6 hpf) during germ layer specification.
- Maintain precise temperature control at 28.5°C throughout illumination.
Monitoring and Validation:
- Image pSmad2 immunostaining at 30-minute intervals to monitor Nodal signaling activity.
- Fix subsets of embryos at shield stage for in situ hybridization of Nodal target genes.
- Track cell internalization movements via time-lapse microscopy of membrane-labeled embryos.
Analysis:
- Quantify pSmad2 nuclear intensity in different embryonic regions relative to illumination pattern.
- Correlate Nodal signaling levels with gene expression patterns and internalization behaviors.
- Compare experimental embryos with dark controls and unmodified siblings.
Table 2: Troubleshooting optoNodal2 Experiments
| Issue | Potential Cause | Solution |
|---|---|---|
| High background signaling in dark | Incomplete sequestration of type II receptor | Verify receptor-cytosolic anchor fusions; optimize expression levels |
| Weak activation response | Suboptimal light intensity or pulse duration | Perform dose-response calibration; increase intensity to 20-40 μW/mm² |
| Spatial pattern blurring | Light scattering in embryonic tissues | Use longer wavelength variants if available; optimize embryo orientation |
| Developmental delays | Excessive phototoxicity or signaling perturbation | Reduce light intensity; shorten illumination duration; include recovery periods |
Protocol 2: Endogenous OptoRhoGEF Manipulation in Drosophila Embryos
This protocol details the use of endogenously tagged OptoRhoGEFs to control cell contractility and internalization during Drosophila gastrulation, enabling quantitative analysis of how mechanical forces shape the embryo.
Materials and Reagents:
- Drosophila embryos with endogenously tagged RhoGEF2-SspB or SspB-Cysts
- UASp>mCherry-iLID-CaaX or UASp>Venus-iLID-CaaX transgenic lines
- Two-photon microscope with 920 nm excitation and 488 nm activation capabilities
- Halocarbon oil 700
- Embryo collection cages with apple juice agar plates
- Standard Drosophila molecular biology reagents
Procedure:
- Embryo Collection and Preparation:
- Cross virgin females from RhoGEF2-SspB or SspB-Cysts lines to males carrying UASp>mCherry-iLID-CaaX.
- Collect 0-2 hour old embryos on apple juice agar plates.
- Dechorionate embryos in 50% bleach for 2 minutes, then rinse thoroughly with water.
- Mount embryos in halocarbon oil on gas-permeable membrane dishes.
Two-Photon Optogenetic Activation:
- Identify embryos at pre-gastrulation stages (stages 4-5) using brightfield microscopy.
- Define regions of interest for RhoGEF activation based on morphological landmarks.
- Set two-photon activation parameters (920 nm, 10-20 mW) with 488 nm recruitment laser.
- Apply light pulses with specific durations (15-60 seconds) and intervals (2-5 minutes).
Live Imaging and Analysis:
- Acquire time-lapse sequences of myosin-GFP or other cytoskeletal markers.
- Track cell shape changes and apical surface areas using segmentation software.
- Quantify furrow formation dynamics and tissue bending angles.
- Correlate light dosage with morphological outcomes.
Validation Experiments:
- Fix and stain embryos for phosphorylated myosin and actin to validate contractility.
- Compare phenotypic strength between OptoGEF2 and OptoCysts activation.
- Assess viability and development of manipulated embryos to adulthood.
Signaling Pathways and Workflows
The following diagrams illustrate key signaling pathways and experimental workflows for implementing light-inducible systems in gastrulation research.
OptoNodal2 Signaling Pathway
Diagram 1: OptoNodal2 Signaling Pathway. This diagram illustrates the light-controlled Nodal signaling pathway using the optoNodal2 system. Blue light induces dimerization between Cry2-tagged type I and CIB1N-tagged type II receptors, forming an active receptor complex that phosphorylates Smad2. Phosphorylated Smad2 then induces expression of Nodal target genes, ultimately promoting cell internalization during gastrulation [6].
Endogenous OptoRhoGEF Activation Workflow
Diagram 2: Endogenous OptoRhoGEF Activation. This workflow depicts the light-controlled activation of endogenous RhoGEFs using the iLID/SspB system. Blue light induces a conformational change in membrane-localized iLID, leading to heterodimerization with SspB-tagged endogenous RhoGEFs and their recruitment to the plasma membrane. At the membrane, RhoGEFs activate Rho GTPases, stimulating actomyosin contractility that drives epithelial furrowing and bending during gastrulation [17].
Research Reagent Solutions
Table 3: Essential Research Reagents for Light-Inducible Systems
| Reagent Category | Specific Examples | Function and Application | Key Characteristics |
|---|---|---|---|
| Photosensitive Domains | LOV2, Cry2, PhoCl, iLID | Light sensing and signal initiation | Specific spectral properties; well-characterized photocycles; minimal dark activity |
| Transcriptional Activators | VP64, p65, Rta, VPR fusions | Transcriptional activation when recruited to DNA | Strong activation potency; minimal toxicity; compatibility with fusion partners |
| Membrane Anchors | CaaX box, transmembrane domains | Localizing optogenetic components to specific cellular compartments | Strong membrane association; proper orientation of fused domains |
| Fluorescent Reporters | GFP, mCherry, mMaple, tagRFP-T | Visualizing expression patterns and protein localization | Brightness; photostability; spectral separation; minimal oligomerization |
| Nuclear Localization/Export Signals | SV40 NLS, HIV Rev NES | Controlling subcellular localization of optogenetic components | Balanced strength for controlled distribution; minimal interference with protein function |
| Model Organisms | Zebrafish, Drosophila, mammalian cell lines | Providing biological context for optogenetic applications | Genetic tractability; optical accessibility; relevance to gastrulation studies |
Light-inducible systems for controlling gene expression have revolutionized our ability to interrogate developmental processes with unprecedented precision. The technologies described in this application note—from the potent DEL-VPR system for mammalian cells to the specialized optoNodal2 and endogenous OptoRhoGEF tools for developmental models—provide researchers with powerful methodologies for dissecting the mechanisms governing cell internalization during gastrulation.
As these technologies continue to evolve, we anticipate several exciting directions for future advancement. The development of multi-color optogenetic systems will enable independent control of multiple signaling pathways simultaneously, allowing researchers to probe the complex interactions between different morphogen systems during gastrulation. Red-shifted optogenetic tools that respond to longer wavelength light will improve tissue penetration and enable manipulation of deeper embryonic structures with minimal scattering [34] [33]. The integration of real-time feedback control systems will allow for dynamic adjustment of illumination patterns based on live readouts of cell behaviors, creating more physiological perturbation paradigms.
The application of these advanced light-inducible systems to gastrulation research promises to yield fundamental insights into how mechanical forces and signaling pathways integrate to shape the developing embryo. By providing precise spatiotemporal control over gene expression and signaling activity, these tools are helping to unravel the complex choreography of cell movements that transform a simple embryonic sheet into a complex, multilayered organism. As these methodologies become increasingly sophisticated and accessible, they will undoubtedly accelerate our understanding of embryonic development and provide new frameworks for addressing developmental disorders and improving regenerative medicine approaches.
The precise control of protein-protein interactions (PPIs) with high spatiotemporal resolution is a cornerstone of modern cell biology research. Among PPIs, protein homo-oligomerization—the physical interaction between identical proteins—represents a pivotal subset widely exploited in activating intracellular signaling pathways [23]. The emerging optogenetic technology, based on genetically encoded light-sensitive proteins, provides unprecedented opportunities to dissect highly complex signaling networks with unmatched specificity and precision [23]. This application note details methodologies for manipulating protein homo-oligomerization and clustering using light, with specific application to controlling cell internalization processes during gastrulation.
Optogenetic Tools for Light-Gated Homo-oligomerization
Several photosensitive protein domains from plants and microbes have been engineered for optogenetic control in mammalian systems. The table below summarizes the key optogenetic tools suitable for inducing protein homo-oligomerization.
Table 1: Key Optogenetic Tools for Light-Induced Homo-oligomerization
| Optogenetic Tool | Origin | Activation Light | Key Properties | Representative Systems |
|---|---|---|---|---|
| CRY2/CIB1 | Arabidopsis thaliana | Blue light (≈488 nm) | Forms both homo-oligomers and heterodimers with CIB1; CRY2PHR (1-498 aa) undergoes light-induced clustering | CRY2olig, CRY2clust, CRY2high, CRY2low, LARIAT system [23] [35] [36] |
| LOV Domains | Various plants, bacteria, fungi | Blue light | Jα helix undocks from PAS core; light-induced dimerization | VfAU1-LOV, iLID/SspB, iLID + tdSspB [23] |
| Phytochromes | Bacteria, plants | Red/Far-red light | Reversible dimerization with co-factors; conformational switching | Cph1, DrBphP [23] |
| CRY2clust | Engineered CRY2 | Blue light | Enhanced clustering with short peptide tag; superior clustering efficiency | CRY2clust (7-amino acid extension) [35] |
The LARIAT (Light-Activated Reversible Inhibition by Assembled Trap) system is particularly versatile, combining CRY2 with CIB1 oligomers and a multimerization domain to form large protein clusters. This system can sequester GFP-tagged proteins in reversible clusters using an anti-GFP nanobody fused to CRY2 [36].
Quantitative Parameters of Optogenetic Systems
Implementation of these tools requires careful consideration of illumination parameters and system performance characteristics, as detailed in the following table.
Table 2: Quantitative Illumination Parameters and Performance Characteristics
| Application/System | Light Stimulation for Microscopy | Light Stimulation for Cell Culture | Cluster Formation Kinetics | Reversibility |
|---|---|---|---|---|
| CRY2-based Trk Activation | 488 nm laser, 1.27 mW/cm² [23] | 5-s irradiation every 5 min, 470 nm, 1 mW/cm² [23] | Seconds to minutes [23] [35] | Rapid (seconds to minutes) [35] |
| CRY2-based FGFR Activation | Laser intensity 1–50 μW (1.30–64.94 mW/cm²) [23] | LED: 5.5 μW of 488 nm for immunoblot; 25 μW, 5 min intervals for viability [23] | Within seconds [35] | Variable by system [23] |
| VfAu-LOV System | 470 nm, 1.7-2.5 μW/mm² [23] | 470 nm (1.7-2.5 μW/mm²) for 8h; 488 nm for 1h for morphology [23] | Seconds [23] | Rapid [23] |
| CRY2clust | Standard blue light illumination [35] | Standard blue light illumination [35] | Within seconds [35] | Rapid upon light termination [35] |
| LARIAT in S2 Cells | Blue light exposure [36] | Blue light exposure [36] | Rapid (within seconds) [36] | Reversible [36] |
Experimental Protocols
Protocol: CRY2clust Implementation for Rapid Protein Oligomerization
Purpose: To induce rapid, efficient homo-oligomerization of target proteins using the CRY2clust system.
Reagents:
- Plasmid encoding CRY2clust (CRY2PHR with 7-amino acid C-terminal extension)
- Appropriate expression vector for target protein fusion
- Cell culture reagents and transfection reagents
- Blue light illumination system (LED array or laser)
Procedure:
- Molecular Cloning: Fuse your protein of interest to the CRY2clust module. The C-terminal positioning is critical for optimal clustering efficiency [35].
- Cell Transfection: Transfect target cells using standard methods. For difficult-to-transfect cells, use viral delivery systems.
- Expression Optimization: Titrate DNA concentration to achieve optimal expression levels (typically 0.5-2 μg DNA per 35mm dish). High concentrations may cause basal clustering.
- Light Stimulation: Illuminate cells with blue light (470-488 nm) at intensities between 1-10 mW/cm². Pulse light stimulation (e.g., 5-second pulses every 5 minutes) can maintain clusters while minimizing phototoxicity [23].
- Monitoring: Visualize cluster formation using live-cell imaging with appropriate fluorescent tags. Clusters should form within seconds of illumination [35].
- Reversibility Testing: Terminate light stimulation and monitor cluster dissipation. Full reversal typically occurs within minutes in dark conditions [35].
Technical Notes:
- The hydrophobicity at position 7 of the C-terminal extension is critical for superior clustering efficiency [35].
- CRY2clust shows significantly enhanced clustering compared to wild-type CRY2PHR across various expression levels [35].
- For sustained activation, consider pulsed illumination regimens rather than continuous light to reduce photodamage.
Protocol: LARIAT System for Protein Sequestration
Purpose: To reversibly trap and inactivate GFP-tagged proteins using light-induced clustering.
Reagents:
- pCMV-CIBN-mCerulean-MP (CIBN fused to multimerization domain)
- pCMV-CRY2-VHH (CRY2 fused to anti-GFP nanobody)
- Cell culture reagents and transfection reagents
- Blue light illumination system
Procedure:
- System Assembly: Co-transfect cells with both CIBN-MP and CRY2-VHH constructs. For consistent expression, use bicistronic vectors or careful ratio optimization (typically 1:1 DNA ratio) [36].
- Expression Induction: Induce expression following system-specific protocols (e.g., heat shock for Hsp70 promoter-driven expression in Drosophila S2 cells) [36].
- GFP-Tagged Protein Expression: Express GFP-tagged target protein (e.g., Mps1, Lgl, or other proteins of interest).
- Light Activation: Expose cells to blue light (470 nm) to induce CRY2-CIBN interaction and cluster formation.
- Functional Assessment: Monitor target protein function pre- and post-clustering. For example:
- Reversibility Testing: Return cells to dark conditions and monitor protein redistribution and functional recovery.
Technical Notes:
- The LARIAT system is particularly effective for proteins where mislocalization inhibits function [36].
- Optimal results require balancing expression levels of all components to minimize basal clustering.
- This system has been successfully adapted for Drosophila S2 cells, providing a powerful platform for genetic screening [36].
Protocol: Application to Gastrulation Models
Purpose: To manipulate cell internalization during gastrulation using optogenetic control of key developmental regulators.
Reagents:
- 2D gastruloid model system [1]
- Optogenetic constructs targeting mesodermal regulators
- Live-cell imaging setup with controlled illumination
- Medium supporting extended culture (beyond 2 days) [1]
Procedure:
- Model System Establishment: Culture 2D gastruloids according to established protocols [1]. The improved extended culture model enables observation of mesoderm migration patterns.
- Target Identification: Identify key proteins involved in mesodermal cell migration during gastrulation. Potential targets include receptors, signaling molecules, or cytoskeletal regulators.
- Optogenetic Tool Selection: Choose appropriate optogenetic system based on desired kinetics and reversibility (CRY2clust for rapid clustering, LARIAT for protein sequestration).
- Integration and Expression: Introduce optogenetic constructs into gastruloid system via transfection or viral transduction.
- Spatiotemporal Activation: Apply patterned light illumination to specific regions of the gastruloid to locally activate or inhibit target proteins during critical developmental windows.
- Phenotypic Analysis: Monitor:
- Mesoderm cell migration patterns (directionality, speed)
- Formation of multi-layered structures
- Expression of mesodermal subtype markers [1]
- Validation: Compare effects to known genetic perturbations and assess reversibility upon light withdrawal.
Technical Notes:
- The extended gastruloid culture model enables observation of mesoderm migration from the edge to the center of the cell group, revealing directional cues [1].
- Precision illumination can target specific subpopulations of cells to create controlled patterns of gene expression or protein activity.
- This approach allows dissection of cell autonomy in gastrulation processes with minimal perturbation to overall development.
Signaling Pathways and Experimental Workflows
The following diagrams illustrate key signaling pathways and experimental workflows for optogenetic control of protein clustering.
Diagram 1: Experimental Workflow for Gastrulation Control
Diagram 2: Optogenetic Signaling Pathways
Research Reagent Solutions
The following table details essential reagents and tools for implementing optogenetic protein clustering experiments.
Table 3: Essential Research Reagents for Optogenetic Protein Clustering
| Reagent/Tool | Function | Example Applications | Key Features |
|---|---|---|---|
| CRY2clust Plasmids | Enhanced blue light-induced clustering | Rapid activation of signaling pathways, receptor oligomerization | Superior clustering efficiency, minimal basal activity [35] |
| LARIAT System Components (CIBN-MP, CRY2-VHH) | Sequestration of GFP-tagged proteins | Functional inhibition of specific proteins, pathway disruption | Versatile (any GFP-tagged target), high spatiotemporal control [36] |
| Optimized FPs for CRY2 (EYFP, Ypet, DsRed) | Fusion partners for CRY2 systems | Optimized clustering efficiency with different fluorescent tags | Defined oligomeric states influence clustering efficiency [35] |
| Extended Gastruloid Culture System | Model for gastrulation studies | Mesoderm migration analysis, cell internalization studies | Enables observation beyond 2 days, reveals directional migration [1] |
| Custom Illumination Systems | Precise light delivery | Spatiotemporal control of protein activity | LED arrays, patterned illumination, intensity control [23] |
Applications in Gastrulation Research
The manipulation of protein homo-oligomerization with light provides unique opportunities to study gastrulation processes with unprecedented precision. Key applications include:
Controlling Mesoderm Migration: Precise activation of guidance receptors or signaling molecules can steer mesoderm cell movements during primitive streak formation [1]. The extended gastruloid model shows mesoderm stem cells traveling from the edge to the center of the cell group, directed by unknown cues that can now be systematically tested [1].
Regulating Cell Internalization: Light-controlled clustering of adhesion molecules or cytoskeletal regulators can modulate the epithelial-mesenchymal transition processes critical for proper gastrulation.
Spatiotemporal Patterning: Patterned illumination can create controlled domains of protein activity, enabling dissection of position-dependent signaling in body plan establishment.
Metabolic Coupling: Recent findings that metabolic signals control developmental tempo through metabolites like FBP suggest opportunities for coupling optogenetic control with metabolic manipulation [37].
Troubleshooting and Optimization
- Basal Clustering in Dark: Reduce expression levels or use CRY2 mutants with reduced dark activity (CRY2low) [23].
- Poor Clustering Efficiency: Ensure C-terminal positioning of tags, consider CRY2clust system, or optimize illumination parameters [35].
- Phototoxicity: Implement pulsed illumination regimens rather than continuous light, reduce intensity, or use red-shifted systems like phytochromes [23].
- Incomplete Functional Inhibition: For LARIAT system, optimize CIBN-MP to CRY2-VHH expression ratios and verify GFP-tagged protein localization [36].
- Variable Gastruloid Responses: Standardize culture conditions and implement precise patterning of light stimulation to account for position effects [1].
The protocols and systems described herein provide a robust foundation for manipulating protein interactions with light, offering powerful approaches for interrogating the dynamic processes of gastrulation with high spatiotemporal precision.
The process of gastrulation, during which a homogeneous sheet of cells folds into the organized three axes of the body plan, represents a pivotal moment in human development. Traditional studies have been hindered by the inaccessibility of the human embryo in utero. Recent breakthroughs in synthetic embryo models and optogenetics now provide an unprecedented window into this process. This case study details the application of light-inducible gene expression to control the morphogen Bone Morphogenetic Protein 4 (BMP4) within synthetic human embryo models, revealing a fundamental interdependence between biochemical signaling and physical mechanical forces that guides embryonic patterning [38]. These findings are framed within a broader thesis on controlling cell internalization during gastrulation, demonstrating that mechanical cues are not merely a consequence but an active driver of cell fate and tissue organization.
Core Discovery: The Interplay of BMP4 Signaling and Mechanical Forces
The seminal finding from the Brivanlou lab established that the activation of BMP4 signaling, while necessary, is insufficient to initiate the formation of the body axes. Using a light-inducible system to trigger BMP4 expression with high spatiotemporal precision, researchers discovered that the transformation begins only when the cells are also subjected to the correct mechanical conditions within their microenvironment [38]. This interplay is critical for breaking symmetry and establishing the head/tail, ventral/dorsal, and right/left axes during gastrulation. The study positions mechanical forces as an essential co-factor with biochemical signals, working in concert to direct the large-scale morphological changes and cell fate decisions that characterize early human development.
Quantitative Data on BMP4 Signaling and Mechanical Cues
BMP4 Signaling Dynamics and Effects
Table 1: Quantitative effects of BMP4 perturbation in model systems.
| Model System | Perturbation | Key Quantitative Outcome | Biological Impact |
|---|---|---|---|
| Mouse Diabetic Model In Vivo [39] | Bmp4 transgenic induction | Significant increase in ECM proteins (Col4), mesangial expansion, and albuminuria | Recapitulation of diabetic nephropathy pathologies |
| Mouse Extra-Embryonic Ectoderm (ExE) [40] | Early ExE-derived BMP4 signal | Necessary for proper mesoendoderm bifurcation, allantois, and PGC specification | Biphasic regulation; later BMP4 restricts PGC pool size |
| hESC Fate Specification [41] | Combinatorial Activin/BMP4 | Relative concentration dictates trajectory choice to definitive endoderm | Direct route vs. indirect route via mesoderm progenitor |
Mechanical Properties of Tissues and Matrices
Table 2: Key mechanical properties and their roles in stem cell behavior.
| Mechanical Property | Definition & Measurement | Role in Stem Cell Behavior and Development |
|---|---|---|
| Stiffness/Elastic Modulus (E) [42] | Resistance to deformation; measured in Pascals (Pa). Slope of stress-strain curve. | Regulates stem cell differentiation and self-renewal. Softer matrices often maintain potency, while stiffer matrices drive specific lineages. |
| Viscoelasticity [42] | Combination of solid (energy-storing) and liquid (energy-dissipating) behaviors. | Allows matrix remodeling by cells without force buildup. Stress relaxation influences cell spreading and differentiation. |
| Cell-Generated Forces [42] | Intrinsic forces (1–3 nN/μm²) exerted via actomyosin contractility on ECM and neighbors. | Drives embryonic morphogenesis, including axis elongation, gastrulation, and epithelial invagination. |
Experimental Protocols
Protocol: Spatiotemporal Optogenetic Control of BMP4 in Organoids
This protocol enables the precise, light-dependent activation of the BMP4 gene to study its role in patterning within a 3D organoid model [43].
System Engineering:
- Tool Selection: Employ a light-inducible CRISPR-Cas9-based transcription system (e.g., SCPTS) or a light-inducible Cre-LoxP system.
- Genetic Construct: Clone the BMP4 coding sequence downstream of a synthetic light-responsive promoter (e.g., CaSP1) or within a LoxP-STOP-LoxP cassette.
Cell Line Generation:
- Stem Cell Culture: Maintain human pluripotent stem cells (hPSCs) in feeder-free conditions using standard culture media.
- Stable Integration: Use lentiviral transduction or PiggyBac transposition to stably integrate the optogenetic BMP4 construct and the necessary effector proteins (e.g., dCas9 split domains, MerCreMer) into hPSCs.
- Selection & Cloning: Apply appropriate antibiotics for 7-10 days to select for successfully transduced cells. Isolate single-cell clones and validate them via PCR and functional testing.
Organoid Formation and Photostimulation:
- Aggregation: Harvest engineered hPSCs and aggregate them into 3D structures using low-attachment U-bottom plates or by embedding in Matrigel droplets.
- Immobilization: For spatial stimulation, embed organoids in a gel droplet (e.g., Matrigel) on a glass-bottom dish to prevent movement during illumination [43].
- Light Patterning:
- Setup: Use a laser scanning confocal microscope or a Digital Micromirror Device (DMD) setup capable of programming complex Region-of-Interest (ROI) illumination patterns.
- Stimulation: Program the system to illuminate specific ROI(s) within the organoid with pulsed blue light (e.g., 450-490 nm) for 10-16 hours [43]. The exact parameters (intensity, pulse frequency) require optimization.
Post-Stimulation Analysis:
- Microdissection: Carefully retrieve the photostimulated organoids from the gel.
- Downstream Assays: Fix for immunostaining, dissociate for single-cell RNA sequencing, or process for spatial transcriptomics to analyze the molecular and morphological consequences of localized BMP4 activation.
Protocol: Assessing the Role of Mechanical Context in BMP4-Driven Patterning
This protocol assesses how the mechanical properties of the substrate influence the cellular response to optogenetically induced BMP4 [42] [38].
Fabrication of Tunable Hydrogels:
- Material Selection: Use synthetic hydrogels like polyacrylamide or polyethylene glycol (PEG), which allow independent control of biochemical and mechanical properties.
- Stiffness Modulation: Vary the crosslinker density to create hydrogel substrates with a range of elastic moduli (e.g., from ~0.5 kPa to ~50 kPa) to mimic different tissue compliances [42].
- Functionalization: Coat the hydrogel surfaces with ECM proteins (e.g., fibronectin, collagen) at a constant density to ensure uniform cell adhesion and integrin signaling across stiffness conditions.
Cell Seeding and Culture:
- Plating: Seed the optogenetically engineered hPSCs (from Protocol 4.1) onto the fabricated hydrogels at a defined density.
- Pre-Conditioning: Culture cells for 24-48 hours to allow them to adapt to the mechanical properties of the substrate.
Uniform Optogenetic Stimulation:
- Setup: Use a programmable LED array board placed inside the cell culture incubator to provide uniform blue light illumination to the entire culture surface.
- Stimulation: Illuminate cells with a defined light regimen to induce BMP4 expression uniformly across all stiffness conditions.
Quantification of Mechano-Chemical Response:
- Immunofluorescence: Fix cells at specific time points post-stimulation and stain for phospho-Smad1/5/8 (direct readout of BMP4 pathway activation), markers of mesoderm/endoderm differentiation (e.g., Brachyury, SOX17), and cytoskeletal organization (F-actin).
- Traction Force Microscopy (TFM): Plate cells on hydrogels embedded with fluorescent beads. Image bead displacements before and after cell detachment to quantify the contractile forces cells exert on their substrate in response to BMP4 signaling [42].
- Gene Expression Analysis: Perform qPCR or RNA-seq to quantify the expression of BMP4 target genes and lineage-specific markers across the different stiffness conditions.
Signaling Pathways and Experimental Workflows
Optogenetic BMP4 Signaling and Mechanical Feedback Loop
Experimental Workflow for Optogenetic Force Probing
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key research reagents and tools for optogenetic mechanobiology studies.
| Category | Reagent / Tool | Function and Application in the Protocol |
|---|---|---|
| Optogenetic Systems | Light-inducible CRISPRa (SCPTS) [43] | Blue light-induced dimerization reconstitutes dCas9 to activate the BMP4 gene from a synthetic promoter. |
| Light-inducible Cre-LoxP [43] | Blue light-induced Cre recombinase excises a STOP cassette, allowing BMP4 expression. | |
| Synthetic Embryo Models | hPSCs (Human Pluripotent Stem Cells) [44] | The starting cell population capable of self-organizing into 2D micropatterns or 3D organoids that model gastrulation. |
| Matrigel / Basement Membrane Extract [43] | Used to support 3D organoid growth and for embedding organoids during spatial photostimulation. | |
| Tunable Matrices | Polyacrylamide or PEG Hydrogels [42] | Synthetic hydrogels with definable stiffness (elastic modulus) to decouple mechanical from biochemical cues. |
| Mechanical Measurement | Traction Force Microscopy (TFM) [42] | A technique to quantify the contractile forces that cells exert on their underlying substrate. |
| Atomic Force Microscopy (AFM) [42] | A method to probe the nanoscale stiffness (elastic modulus) of cells and their immediate microenvironment. | |
| Key Reagents | Recombinant BMP4 Protein [41] | Used for control experiments and for validating the effects of pathway activation. |
| BMP Signaling Inhibitors (e.g., Dorsomorphin) | Used to confirm the specificity of the BMP4-activated phenotype. | |
| ROCK Inhibitor (Y-27632) [42] | Used to inhibit actomyosin-based contractility, thereby probing the role of cell-generated forces. | |
| Detection Assays | Anti-pSmad1/5/8 Antibodies [39] | For immunofluorescence detection of activated BMP/Smad signaling pathway. |
| scRNA-seq / Spatial Transcriptomics [43] | For unbiased profiling of cell states and gene expression patterns in response to localized BMP4 and mechanical cues. | |
| 6,7-Dihydro-5H-cyclopenta[b]pyridin-5-ol | 6,7-Dihydro-5H-cyclopenta[b]pyridin-5-ol, CAS:1065609-70-2, MF:C8H9NO, MW:135.16 g/mol | Chemical Reagent |
| (1-(4-Iodophenyl)cyclobutyl)methanamine | (1-(4-Iodophenyl)cyclobutyl)methanamine, CAS:1936255-32-1, MF:C11H14IN, MW:287.14 g/mol | Chemical Reagent |
Gastrulation is a fundamental and dramatic process in early development, where a uniform sheet of cells transforms into a multi-layered structure, establishing the basic body plan of an organism [45]. The study of human gastrulation presents significant ethical and technical challenges, as it occurs after implantation and is largely inaccessible to direct observation [7] [46]. Recent advances in stem cell technology have provided unprecedented insights through the development of engineered models that replicate aspects of this critical developmental window. These include pre-gastrulation models like blastoids, gastrulation models such as 2D micropatterned systems and 3D gastruloids, and post-gastrulation models that mimic early somitogenesis and axial elongation [7].
Concurrently, breakthroughs in optogenetics and light-controlled genetic tools have created unprecedented opportunities for precise spatiotemporal manipulation of developmental signaling pathways [47] [3]. This convergence of model system development and precision control technology enables researchers to dissect the complex interplay between biochemical signaling and mechanical forces that guide gastrulation with unprecedented precision. These approaches are particularly valuable for investigating cell internalization during gastrulation – the process by which cells move from the epithelial layer to the interior of the embryo to form new germ layers.
This application note provides a practical workflow for integrating these advanced technologies, focusing specifically on the use of light-based tools to control cell internalization during gastrulation. We outline key methodologies, experimental protocols, and analytical approaches that leverage the precision of optogenetic control to unravel the mechanisms governing this pivotal developmental process.
Tool Selection: Optogenetic Systems for Gastrulation Research
Selecting the appropriate optogenetic system is fundamental to designing experiments aimed at controlling cell internalization during gastrulation. The optimal choice depends on the specific biological question, whether it involves activating or inhibiting specific signaling pathways, controlling gene expression, or manipulating mechanical forces. Below, we compare the primary optogenetic systems applicable to gastrulation studies.
Table 1: Comparison of Key Optogenetic Tools for Gastrulation Research
| Tool Name | Type/Mechanism | Key Applications in Gastrulation | Activation Wavelength | Key Advantages |
|---|---|---|---|---|
| PhoBITs [47] | Photo-inducible binary interaction tools | Controlling gene expression, receptor signaling, calcium channels, immune responses, cell death | Blue light (~450-490 nm) | Compact size, versatility, can be configured as ON or OFF switches |
| Optogenetic BMP4 [3] | Light-activated signaling pathway activation | Precise symmetry breaking, germ layer specification, studying BMP4 role in gastrulation initiation | Customizable (system-dependent) | Enables precise spatial patterning of morphogen signaling |
| LEVA [48] | Light-induced extracellular vesicle/particle adsorption | Patterning cellular messengers, studying cell guidance and communication during morphogenesis | Ultraviolet light | Controls extracellular microenvironment rather than intracellular pathways |
Guidance for Tool Selection
- For Pathway Activation/Inhibition Studies: PhoBITs offer exceptional versatility for controlling specific protein interactions and downstream signaling events with high temporal precision [47]. Their compact size minimizes functional disruption to native proteins.
- For Spatial Pattern Control: The optogenetic BMP4 system provides unparalleled control over morphogen gradient establishment, crucial for studying symmetry breaking and axial patterning [3].
- For Microenvironment Engineering: LEVA technology enables precise arrangement of extracellular vesicles and particles to study how native cellular messengers guide cell behavior during gastrulation and tissue organization [48].
Core Experimental Workflows
This section details practical protocols for establishing and utilizing gastrulation models with integrated optogenetic control systems.
Protocol 1: Generating Gastruloids from Human Pluripotent Stem Cells (hPSCs)
Gastruloids are 3D aggregates of embryonic stem cells that undergo key events of early mammalian development, including symmetry breaking and germ layer specification [45].
Materials:
- Naive human pluripotent stem cells (e.g., RUES2 line) [46]
- AggreWell plates (for controlled aggregation)
- PALLY-LY medium [46]
- Laminin-521 substrate
- ROCK inhibitor (Y-27632)
- Extracellular matrix (e.g., 5% Geltrex)
Procedure:
- Cell Preparation: Culture naive hPSCs under conditions that maintain their pre-implantation epiblast state, characterized by dome-shaped colonies expressing markers like KLF17 and lacking post-implantation markers (CD24, SSEA4) [46].
- Aggregation: Transfer defined numbers of naive hPSCs to AggreWell plates containing PALLY-LY medium to promote self-organization into 3D blastoids.
- Maturation: Culture aggregates for 5-7 days, during which they should develop distinct lineages: epiblast (NANOG+, OCT4+), primitive endoderm (GATA4+, SOX17+), and trophectoderm (GATA3+) [46].
- In Vitro Attachment: Plate blastoids on laminin-521-coated substrates with ROCK inhibitor and extracellular matrix supplement to promote attachment and further development.
- Validation: At 7-10 days post-attachment (dpa), assess primitive streak formation via immunostaining for OCT4+/BRA+ co-expression [46].
Quality Control: Only proceed with blastoids that show clear cavitation and distinct lineage segregation. Typically, 8-52% of attached blastoids will initiate primitive streak formation between 7-10 dpa [46].
Protocol 2: Integrating PhoBITs for Light-Controlled Signaling in Gastruloids
PhoBITs enable precise, light-dependent control of protein interactions to manipulate key signaling pathways during gastrulation events [47].
Materials:
- PhoBIT plasmids (PhoBIT1: light-OFF switch; PhoBIT2: light-ON switch) [47]
- Lentiviral or transfection system for stable cell line generation
- Blue light illumination system (LED array with ~450-490 nm wavelength)
- Customizable light patterning device (for spatial control)
Procedure:
- Cell Engineering:
- Introduce PhoBIT constructs into hPSCs using lentiviral transduction or electroporation.
- Generate stable cell lines expressing PhoBIT-tagged proteins of interest (e.g., signaling receptors, transcription factors).
- Validate proper localization and function of PhoBIT-tagged proteins without light activation.
Gastruloid Formation:
- Aggregate PhoBIT-expressing hPSCs following Protocol 1.
- During appropriate developmental windows, apply blue light illumination according to experimental design.
Light Activation Paradigms:
- For temporal studies: Apply uniform blue light pulses of varying duration and frequency.
- For spatial patterning: Use digital micromirror devices to project specific light patterns onto gastruloids to create controlled signaling domains.
Phenotypic Analysis:
- Monitor cell internalization events via live imaging.
- Fix samples at specific timepoints for immunostaining of germ layer markers.
- Process samples for single-cell RNA sequencing to assess transcriptomic changes.
Application Example: To test the role of specific signaling pathways in mesoderm formation, engineer hPSCs with PhoBIT-tagged activators of Wnt or Nodal signaling. Apply localized light activation to specific regions of gastruloids and assess BRA expression and mesoderm formation [47].
Protocol 3: Optogenetic Control of BMP4 Signaling for Symmetry Breaking
This protocol uses light to activate BMP4 signaling with precise spatial and temporal control, enabling dissection of its role in the initial stages of gastrulation [3].
Materials:
- Optogenetic BMP4 hPSC line [3]
- Custom microchip platforms or engineered microenvironments
- Confinement substrates (e.g., tension-inducing hydrogels)
- Blue light illumination system
Procedure:
- Model Setup:
- Plate optogenetic BMP4 hPSCs on configured substrates (unconfined vs. confined).
- For mechanical confinement studies, embed cells in tension-inducing hydrogels.
Light Activation:
- Apply specific wavelengths of light to activate BMP4 signaling in precise patterns (e.g., at colony edges).
- Vary illumination parameters (intensity, duration, pattern) across experimental groups.
Mechanical Force Modulation:
- Compare outcomes between low-tension (unconfined) and high-tension (confined) environments.
- Assess nuclear localization of YAP1 as a readout of mechanical tension.
Analysis:
- Evaluate symmetry breaking by assessing the emergence of BRA+ territories.
- Monitor downstream signaling pathways (WNT, Nodal) activation.
- Assess germ layer specification through marker expression (e.g., SOX17 for endoderm, BRA for mesoderm).
Key Insight: Research shows that BMP4 signaling alone is insufficient to drive complete gastrulation; proper mechanical tension is also required. Only when BMP4 activation occurs in confined, tension-inducing environments do gastruloids robustly form mesoderm and endoderm layers [3].
Analytical Methods for Gastrulation Studies
Live Imaging and Quantitative Analysis of Gastrulation Dynamics
Real-time observation of gastrulation events provides crucial insights into the dynamics of cell internalization and movement.
Materials and Setup:
- Light-sheet microscopy system [49]
- Environmental chamber for maintaining physiological conditions (temperature, COâ‚‚, humidity)
- Specimen holders that support embryos without agarose embedding
- Computational tools for 4D data analysis and cell tracking
Procedure:
- Sample Preparation: Transfer gastruloids to specialized holders that maintain viability without constraining development.
- Image Acquisition: Use digital scanned light-sheet microscopy (DSLM) to capture 3D time-lapse datasets over 12-hour periods, minimizing photodamage.
- Cell Tracking: Employ manual and semiautomatic tracking programs to analyze cell trajectories and movements from the 4D datasets.
- Quantitative Analysis: Extract parameters including cell speed, directionality, division patterns, and internalization events.
This protocol enables tracking of individual cells during gastrulation, allowing direct observation of epithelial-to-mesenchymal transition and cell internalization events that are fundamental to germ layer formation [49].
Single-Cell RNA Sequencing for Molecular Profiling
scRNA-seq provides comprehensive transcriptomic data from developing gastruloids, enabling detailed characterization of cell states and lineages.
Procedure:
- Sample Collection: Harvest gastruloids at specific timepoints (e.g., 7 dpa, 10 dpa).
- Cell Dissociation: Gently dissociate into single-cell suspensions while maintaining viability.
- Library Preparation: Use standard scRNA-seq protocols (e.g., inDrops, 10X Genomics).
- Bioinformatic Analysis:
- Perform clustering to identify distinct cell populations.
- Annotate clusters based on marker gene expression (e.g., BRA for primitive streak, SOX17 for endoderm).
- Conduct pseudotime analysis to reconstruct developmental trajectories.
This approach typically identifies 16+ distinct cell clusters representing derivatives of all three germ layers and extraembryonic cell types, providing validation of gastruloid developmental progression [46].
Essential Research Reagent Solutions
Successful implementation of optogenetic gastrulation studies requires specific reagents and tools. The table below details essential materials and their functions.
Table 2: Essential Research Reagents and Materials for Optogenetic Gastrulation Studies
| Category | Specific Reagent/Tool | Function/Application | Key Considerations |
|---|---|---|---|
| Stem Cell Lines | Naive hPSCs (e.g., RUES2) [46] | Generate gastruloids with enhanced self-organization capacity | Verify naive state markers (KLF17/SUDS2); absence of primed markers |
| Optogenetic BMP4 hPSCs [3] | Enables light-controlled activation of BMP4 signaling | Confirm tight light-dependent control of BMP4 pathway activation | |
| Optogenetic Tools | PhoBIT plasmids [47] | Light-controlled protein-protein interactions | Choose between PhoBIT1 (light-OFF) and PhoBIT2 (light-ON) configurations |
| Culture Systems | AggreWell plates [46] | Forms uniformly sized 3D cell aggregates | Optimize cell number per well for consistent gastruloid formation |
| Laminin-521 coating [46] | Promotes in vitro attachment of blastoids | Test alternative coatings (e.g., Matrigel) for specific applications | |
| Signaling Modulators | ROCK inhibitor (Y-27632) [46] | Enhances cell survival after dissociation and plating | Use during initial attachment phase only; wash out for continued culture |
| Geltrex/ECM matrix [46] | Provides structural support and biochemical cues | Concentration optimization required for desired mechanical properties | |
| Imaging Tools | Light-sheet microscope [49] | Live imaging of gastrulation dynamics with minimal phototoxicity | Requires specialized equipment and computational analysis capabilities |
| Blue light illumination systems [47] [3] | Activates optogenetic components | Configure for uniform illumination or patterned light delivery |
Signaling Pathways and Experimental Workflows
The following diagrams illustrate key signaling pathways and experimental workflows for optogenetic gastrulation studies, created using DOT language and compliant with the specified color and formatting guidelines.
Mechanical Force and Biochemical Signaling Integration in Gastrulation
Diagram Title: Mechanical and Biochemical Control of Gastrulation
Experimental Workflow for Optogenetic Gastrulation Studies
Diagram Title: Optogenetic Gastrulation Study Workflow
PhoBITs Molecular Mechanism
Diagram Title: PhoBITs Molecular Switching Mechanism
The integration of advanced gastrulation models with precise light-based control technologies represents a transformative approach for developmental biology research. The workflows and protocols outlined in this application note provide a practical framework for investigating the mechanisms of cell internalization during gastrulation with unprecedented spatiotemporal precision. By leveraging these tools, researchers can dissect the complex interplay between biochemical signaling and mechanical forces that guide this fundamental developmental process, with potential applications in regenerative medicine, infertility research, and therapeutic development.
Overcoming Technical Hurdles: A Guide to Optimizing Optogenetic Experiments
Long-term live-cell imaging is indispensable for capturing dynamic cellular processes, yet the phototoxicity induced by fluorescent imaging can compromise cell health and introduce experimental artifacts, ultimately confounding data interpretation [50] [51]. This challenge is particularly acute in sensitive research areas such as the study of mechanical forces during gastrulation in early human development, where maintaining physiological conditions is paramount for observing true biological phenomena [38] [29]. This Application Note synthesizes current strategies to mitigate phototoxicity, providing a framework for researchers, scientists, and drug development professionals to design robust long-term imaging experiments. The protocols and data presented herein are designed to be integrated into a broader thesis on controlling cell internalization during gastrulation with light, ensuring that observed dynamics reflect genuine biology rather than light-induced damage.
Core Mechanisms and Assessment of Phototoxicity
Understanding the Source of Damage
Phototoxicity occurs when the absorption of light by cellular components or fluorescent probes generates reactive oxygen species (ROS), leading to oxidative stress and cellular damage [51]. Mitochondria are especially vulnerable, with phototoxicity triggering functional impairments such as the dissipation of mitochondrial membrane potential (( \Delta\psi_m )), dysregulation of intracellular calcium homeostasis, and ultimately, cell death [51]. The cumulative nature of this damage makes it a significant constraint in extended imaging sessions [52].
Quantitative Measurement
Implementing a quantitative assessment of phototoxicity is a critical first step in any live-cell imaging regimen. A method proposed in the literature uses the organisms under study themselves to "reveal the range over which any given fluorescent imaging microscope will yield valid results," thereby identifying a clear threshold for phototoxic damage distinct from photobleaching [50]. Key quantitative markers for assessing mitochondrial phototoxicity include:
- Alterations in ( \Delta\psi_m ): A sensitive and early indicator of mitochondrial dysfunction, often quantified using fluorescent probes like TMRM or JC-1 [51].
- Morphological Analysis: Machine learning and high-content analysis can quantify changes in mitochondrial morphology and network dynamics in response to light exposure [51].
Table 1: Quantitative Assays for Assessing Phototoxicity
| Assay / Method | Measured Parameter | Key Insight |
|---|---|---|
| Threshold Metrology [50] | Cell viability/physiology post-illumination | Identifies a clear light dose threshold between valid results and phototoxic artifacts. |
| Membrane Potential Probes [51] | Mitochondrial membrane potential (( \Delta\psi_m )) | A sensitive, early indicator of functional impairment. |
| PrestoBlue Assay [52] | Overall cell viability/metabolic activity | Used to compare viability across different culture microenvironments under imaging stress. |
| Automated Image Analysis [52] | Network morphology, neurite outgrowth, somata clustering | Quantifies structural indicators of health and maturation over time. |
Strategic Optimization of the Cellular Microenvironment
The in vitro cellular microenvironment plays a crucial role in bolstering cell health and resilience against phototoxic stress. A recent systematic investigation optimized three key culturing conditions for human embryonic stem cell-derived cortical neurons subjected to daily imaging over 33 days [52] [53].
Culture Media Composition
The choice of culture medium is a primary determinant of phototoxic resistance.
- Brainphys Imaging Medium (BPI) significantly outperformed Neurobasal Plus medium (NB) in supporting neuron viability, neurite outgrowth, and self-organisation under fluorescent imaging conditions [52].
- The antioxidant profile of BPI medium, which is rich in light-protective compounds and omits reactive components like riboflavin, is credited for actively curtailing ROS production and protecting mitochondrial health [52].
Extracellular Matrix (ECM) and Seeding Density
- ECM Coating: A synergistic relationship exists between the species-specificity of laminin and the culture media. The combination of NB medium and human-derived laminin was found to reduce cell survival, whereas BPI medium supported robust development with both murine- and human-derived laminin [52].
- Cell Seeding Density: A higher seeding density ((2 \times 10^5) cells/cm²) fostered somata clustering, which can facilitate neuroprotective paracrine signaling. However, in the cited study, it did not significantly extend viability compared to a lower density ((1 \times 10^5) cells/cm²) [52].
Table 2: Optimizing Culture Conditions to Mitigate Phototoxicity
| Culture Parameter | Comparison | Impact on Phototoxicity & Cell Health |
|---|---|---|
| Culture Medium | Brainphys Imaging (BPI) vs. Neurobasal (NB) | BPI medium supports greater viability, outgrowth, and self-organisation; its antioxidant profile mitigates ROS [52]. |
| Extracellular Matrix | Human-derived vs. Murine-derived Laminin | Species-specific synergy with media; NB medium + human laminin reduced survival, while BPI worked well with both [52]. |
| Seeding Density | High ((2 \times 10^5)/cm²) vs. Low ((1 \times 10^5)/cm²) | High density fosters protective somata clustering but did not significantly extend viability over low density in a neuronal context [52]. |
Experimental Protocols
Protocol: Quantitative Assessment of Phototoxicity Threshold
This protocol is adapted from methods designed to measure the phototoxicity of an imaging microscope itself [50].
1. Sample Preparation:
- Culture the cells of interest (e.g., mammalian cell models or microorganisms relevant to your research) under optimal standard conditions.
2. Illumination Gradient:
- Define a range of illumination intensities (laser power or lamp output) and exposure times that span from typical imaging conditions to higher, potentially damaging doses.
- Expose different sample groups to this gradient of total light dose.
3. Viability and Function Assessment:
- For each light dose group, quantitatively assess cell health. This can include:
- Viability Assays: Such as PrestoBlue to measure metabolic activity [52].
- Functional Assays: Such as measuring mitochondrial membrane potential (( \Delta\psi_m )) using fluorescent dyes [51].
- Morphological Analysis: Using automated image analysis pipelines to quantify changes in cell structure or network organization [52].
4. Data Analysis:
- Plot the measured parameter of cell health (e.g., viability percentage) against the total light dose.
- Identify the inflection point where the parameter begins to decline significantly. This is the phototoxicity threshold for your specific experimental setup and cell type, beyond which data may be confounded by artifacts [50].
Protocol: Differentiating Bound from Internalized Particles in Gastrulation Studies
This protocol, leveraging imaging flow cytometry, is critical for studies of internalization during gastrulation, allowing for high-throughput, quantitative discrimination of surface-bound and internalized cargo [54].
1. Sample Preparation and Exposure:
- Expose cells (e.g., gastrulation-relevant progenitors) to the fluorescently tagged particles of interest (e.g., pathogens, synthetic particles, or other cells).
- Incubate under desired conditions to allow for binding and internalization.
2. Fixation and Staining:
- Fix cells with a gentle fixative like 2% PFA without permeabilizing the cell membrane.
- Stain the samples with a particle-specific antibody conjugated to a fluorophore with a distinct emission spectrum from the original particle tag. This antibody will only bind to extracellular, surface-bound particles.
3. Imaging Flow Cytometry:
- Analyze the samples using an imaging flow cytometer.
- Identify single, well-focused cells using brightfield and side-scatter features [55].
- For each cell-associated particle, the software can discriminate:
- Extracellular particles: Display fluorescence from both the original tag and the secondary antibody (double positive).
- Internalized particles: Display fluorescence only from the original tag, as the membrane-impermeant secondary antibody cannot access them (single positive) [54].
4. Quantitative Analysis:
- Use the instrument's software (e.g., IDEAS) to apply an internalization algorithm and spot count feature.
- This provides statistical data on the percentage of particles internalized and the number of particles per cell, offering a robust, high-throughput alternative to manual microscopy analysis [54] [55].
The Scientist's Toolkit: Essential Reagents and Materials
Table 3: Key Research Reagent Solutions for Mitigating Phototoxicity
| Reagent / Material | Function / Application | Specific Example |
|---|---|---|
| Specialized Imaging Media | Provides a rich antioxidant profile and omits light-reactive molecules (e.g., riboflavin) to reduce ROS generation during illumination. | Brainphys Imaging Medium with SM1 system [52]. |
| Physiological Extracellular Matrix | Provides physiological anchorage and bioactive cues that support cell health, maturation, and resilience. | Human-derived LN511 Laminin [52]. |
| Mitochondrial Membrane Potential Probes | Sensitive dyes used to quantitatively assess early-stage phototoxic stress via mitochondrial health. | TMRM, JC-1, Rhodamine derivatives [51]. |
| Cell Viability Assays | Measures metabolic activity as a proxy for overall cell health following light exposure. | PrestoBlue Assay [52]. |
| Imaging Flow Cytometer | Instrument that combines high-throughput flow cytometry with single-cell imaging, ideal for quantifying internalization events. | Systems like the ImageStreamX [54] [55]. |
| 5-Iodomethyl-2-methyl-pyrimidine | 5-Iodomethyl-2-methyl-pyrimidine, CAS:2090297-94-0, MF:C6H7IN2, MW:234.04 g/mol | Chemical Reagent |
Mitigating phototoxicity is not merely a technical exercise but a fundamental requirement for generating physiologically relevant data in long-term live-cell imaging experiments. By integrating the strategies outlined—quantitatively assessing phototoxicity thresholds, strategically optimizing the cellular microenvironment with specialized media and ECM, and employing robust protocols for differentiating biological processes like internalization—researchers can significantly enhance the validity of their findings. For those investigating complex processes like cell internalization during gastrulation using light-based tools, these protocols provide a foundation for ensuring that the observed mechanical forces and chemical cues are genuine drivers of development, not mere artifacts of illumination [38] [29].
The precise control of cell internalization during gastrulation using light-based research methodologies introduces a fundamental challenge: the trade-off between spatial precision and temporal resolution. High spatial resolution is required to observe and manipulate subcellular events, such as the initiation of apical constriction. Conversely, high temporal resolution is essential to capture the rapid, dynamic sequence of morphogenetic movements. Optimizing this balance is critical for obtaining meaningful data without causing photodamage or missing key transitional states. This document outlines application notes and protocols for navigating this trade-off within the context of gastrulation research, providing a framework for researchers to design robust experiments.
Quantitative Data: Comparing Spatiotemporal Configurations
The choice of imaging modality and parameters directly dictates the possible spatiotemporal balance. The table below summarizes key performance metrics for different configurations relevant to live embryo imaging.
Table 1: Trade-offs in Spatiotemporal Imaging Configurations
| Imaging / Analysis Modality | Spatial Context | Typical Spatial Resolution | Typical Temporal Resolution | Primary Trade-off Consideration |
|---|---|---|---|---|
| Confocal Microscopy | Subcellular to Tissue | High (e.g., ~200 nm lateral) | Low to Medium (seconds to minutes) | Higher spatial resolution increases laser exposure and acquisition time, reducing temporal resolution and increasing phototoxicity [56]. |
| Light Sheet Microscopy | Whole Embryo | Medium to High | High (sub-second to seconds) | Optimized for volumetric imaging over time, but ultimate spatial resolution may be lower than point-scanning confocals. |
| Satellite Earth Observation (Analogy) | Regional Crop Fields | 100 m vs 1 km | 5-day vs Daily | In a remote sensing context, a study on crop yield found that finer spatial resolution (100 m) enabled more accurate yield estimations than higher temporal frequency, highlighting that spatial precision can sometimes outweigh temporal density [57]. |
| Satellite Snow Mapping (Analogy) | Mountain Snowpack | 30 m vs 463 m | 16-day vs Daily | Sensor fusion to create daily, 30 m resolution snow cover maps resulted in lower basin-wide bias in Snow Water Equivalent (SWE) reconstruction compared to daily, coarse-resolution (463 m) data [58]. |
| "Minimal Videos" (Human Recognition) | Object/Action Recognition | Minimal recognizable area | Minimal recognizable duration | Recognition is achieved by efficiently combining spatial and motion cues where each source alone is insufficient; reducing either dimension makes the stimulus unrecognizable [56]. |
Experimental Protocols for Spatiotemporal Analysis in Gastrulation
The following protocols are adapted from recent research on mechanical forces and genetic patterning during gastrulation.
Protocol: Optogenetic Perturbation of Actomyosin Contractility at the Cephalic Furrow
This protocol details a method for spatially and temporally precise inhibition of actomyosin contractility to study its role in gastrulation, as demonstrated in Drosophila melanogaster [59].
1. Key Research Reagent Solutions
Table 2: Essential Reagents for Optogenetic Perturbation
| Item | Function / Explanation |
|---|---|
| Opto-DNRho1 System | An optogenetic tool for the light-controlled, localized inhibition of Rho1 GTPase, a key regulator of actomyosin contractility [59]. |
| eve1KO Mutant Line | A genetically engineered fly line specifically lacking the first stripe of Even-skipped (eve1) expression, used to block cephalic furrow formation without broad developmental defects [59]. |
| Live Imaging Chamber | A chamber that maintains embryo viability (humidity, temperature) while allowing for precise light illumination and high-resolution microscopy. |
| Myosin-II (MyoII) Antibody | For staining and visualizing the accumulation of non-muscle Myosin-II to identify regions of active contractility [59]. |
2. Workflow Diagram: Optogenetic Perturbation and Phenotypic Analysis
Title: Workflow for spatially precise optogenetic perturbation during gastrulation.
3. Step-by-Step Methodology
- Embryo Preparation and Mounting: Collect embryos of the appropriate genotype (e.g., expressing Opto-DNRho1). Dechorionate and mount in a live imaging chamber with halocarbon oil to maintain physiological conditions.
- Spatial Targeting: Using a low-intensity reference light, identify the region of the embryo corresponding to the presumptive cephalic furrow (approximately 33% embryo length). Define a precise region of interest (ROI) for illumination using the microscope's software.
- Temporal Perturbation: At gastrulation onset, initiate a calibrated light pulse to activate the Opto-DNRho1 system only within the predefined ROI. The duration and intensity of the pulse control the strength of the perturbation.
- Concurrent Imaging: Throughout the experiment, acquire high-resolution time-lapse images. A balance must be struck: use sufficient spatial resolution and z-stacks to capture tissue deformation (e.g., buckling) and sufficient temporal resolution to track the dynamics of furrow formation failure.
- Phenotypic Quantification: Analyze the image data for:
- Head-Trunk Buckling: Characterized by abrupt, broad indentation occurring ~9.4 minutes after posterior midgut invagination, with variable dorso-ventral position [59].
- CF Formation Failure: Absence of the characteristic narrow, actively forming cleft with planar polarized MyoII [59].
- Quantitative Metrics: Measure changes in apical cell surface area, tissue curvature, and the timing of deformation events compared to controls.
Protocol: Genetic Dissection of Cephalic Furrow Initiation
This protocol uses genetic mutants to investigate the role of specific transcription factors in patterning the site of cell internalization [59].
1. Workflow Diagram: Genetic Analysis of Gastrulation
Title: Pipeline for genetic analysis of gastrulation initiation.
2. Step-by-Step Methodology
- Sample Generation: Utilize mutant lines such as
eve1KOorbtdmutants. Cross flies to obtain embryos of the desired genotype. - Fixation and Staining: Fix embryos at precise developmental time points. Perform double fluorescent in situ hybridization (FISH) to detect the overlapping expression patterns of
btdandeve1. Co-stain for MyoII to visualize contractile networks. - High-Spatial-Resolution Imaging: Image fixed samples using high numerical aperture (NA) objectives on a confocal microscope. Maximize spatial resolution (e.g., by using higher zoom and smaller pixel sizes) as temporal dynamics are not a factor.
- Analysis: Determine the presence or absence of overlapping
btdandeve1expression domains. Correlate this spatial patterning information with the success or failure of MyoII accumulation and cephalic furrow formation. This establishes the molecular blueprint for the morphogenetic event.
The Scientist's Toolkit: Key Reagent Solutions
A consolidated table of essential reagents and tools for research in controlling cell internalization during gastrulation.
Table 3: Research Reagent Solutions for Gastrulation Studies
| Category / Item | Function in Research |
|---|---|
| Genetic Tools | |
eve1KO mutant |
Specifically blocks cephalic furrow formation by removing a key positional cue, enabling study of its mechanical role [59]. |
btd (Buttonhead) mutant |
Disrupts the genetic program for CF initiation; used to study genetic patterning upstream of morphogenesis [59]. |
| Optogenetic Tools | |
| Opto-DNRho1 system | Allows light-controlled, localized inhibition of actomyosin contractility to test the mechanical function of specific tissues in real-time [59]. |
| Imaging & Analysis | |
| High-sensitivity camera (sCMOS) | Crucial for capturing high-temporal-resolution data with low light, minimizing phototoxicity during live imaging. |
| Custom live imaging chamber | Maintains embryo viability for long-term imaging by controlling temperature and gas exchange. |
| Morphometric analysis software | Quantifies dynamic changes in cell shape, area, and tissue-level forces from time-lapse datasets [59]. |
| Labeling | |
| Myosin-II (MyoII) antibodies | Visualizes the localization and intensity of actomyosin contractility, a key driver of cell internalization [59]. |
mRNA probes for btd, eve |
Maps the spatial domains of gene expression that prefigure and guide morphogenetic events [59]. |
In the field of developmental biology and drug discovery, the use of light to control and observe biological processes has become a powerful tool. This is particularly true for research aimed at controlling cell internalization during gastrulation, a fundamental morphogenetic event in early embryonic development. The establishment of spatial patterns of signaling activity, such as the Nodal morphogen gradient, provides crucial instructions to embryonic cells, guiding them to make appropriate fate decisions [6]. Optogenetics, the rewiring of signaling pathways to respond to light, has emerged as a promising strategy for achieving agile and precise control over developmental gene expression and signaling, effectively converting photons into morphogens [6]. Concurrently, advanced imaging modalities like light sheet fluorescence microscopy (LSFM) are vital for monitoring the outcomes of these interventions, such as drug delivery and the complex cellular sociology within 3D models [60] [61] [62].
However, the very power of these techniques creates a central challenge: the efficient and uniform delivery of light deep within scattering biological tissues and three-dimensional (3D) cell cultures. This application note details the specific challenges and provides validated protocols to overcome them, with a focus on applications in gastrulation research and 3D model systems.
Key Challenges in Light Delivery for 3D and Deep-Tissue Systems
Achieving precise optogenetic control or high-quality imaging in thick samples is hindered by several physical and technical obstacles.
- Light Scattering and Absorption: Cellular components and extracellular matrices in tissues and 3D cultures scatter and absorb photons, leading to rapid degradation of light intensity and resolution with depth. This results in weak optogenetic activation and poor image quality in the core of samples.
- Phototoxicity and Photobleaching: The high light doses often required to penetrate deep into a sample can generate cytotoxic reactive oxygen species, compromising cell viability and normal development, while also bleaching fluorescent signals.
- Spatiotemporal Control and Throughput: Generating complex, dynamic light patterns for optogenetics with high spatial and temporal resolution in multiple live samples simultaneously remains a significant technical hurdle, limiting the statistical power of experiments [6].
The table below summarizes these core challenges and their direct impacts on research:
Table 1: Core Challenges in Light Delivery for 3D and Deep-Tissue Systems
| Challenge | Impact on Research |
|---|---|
| Light Scattering & Absorption | Degrades signal-to-noise ratio in imaging; causes uneven and weak optogenetic stimulation in deep tissue layers. |
| Phototoxicity & Photobleaching | Compromises cell viability and normal developmental processes; limits duration of live-cell imaging experiments. |
| Limited Spatiotemporal Control | Hinders the ability to recreate complex, endogenous morphogen patterns needed to test quantitative models of patterning [6]. |
| Low Throughput | Restricts the number of embryos or organoids that can be processed in parallel, reducing experimental robustness and screening capacity. |
Optimized Solutions and Instrumentation
Advanced Microscopy Modalities
Different imaging techniques offer varying balances of penetration depth, speed, and phototoxicity. The choice of microscope is critical for successful deep tissue and 3D culture work.
Table 2: Comparison of Imaging Modalities for 3D and Deep-Tissue Studies
| Imaging Modality | Principle | Advantages for 3D Cultures | Limitations |
|---|---|---|---|
| Confocal Microscopy | Uses a pinhole to reject out-of-focus light. | High-resolution optical sectioning; widely available. | Limited penetration depth (>200 µm); high phototoxicity and photobleaching; not suitable for studying nanocarrier penetration in large spheroids [62]. |
| Light Sheet Microscopy | Illuminates the sample with a thin sheet of light from the side, capturing emitted light with a perpendicular detector. | Fast acquisition; very low phototoxicity and photobleaching; excellent for long-term live imaging of large, opaque samples like embryos and spheroids; ideal for monitoring drug/nanocarrier penetration [62]. | Lower resolution than confocal; can require sample clearing for very large samples; sample mounting can be complex. |
| Line-Scan Brillouin Microscopy (LSBM) | Measures frequency shift of scattered light to map material properties (longitudinal modulus). | Non-contact mechanical mapping; high spatial and temporal resolution; capable of probing material properties deep within tissues (e.g., inside a Drosophila embryo encased in a vitelline membrane) [8]. | Measures mechanical properties at GHz frequencies, which are not directly equivalent to static moduli; requires specialized equipment and expertise. |
Optogenetic Patterning and High-Throughput Systems
For optogenetic control beyond simple illumination, specialized systems are required. An effective solution is an ultra-widefield patterned illumination platform that allows for spatial light patterning and live imaging of up to 36 zebrafish embryos in parallel [6]. This system addresses the throughput bottleneck and enables systematic exploration of morphogen signaling patterns by providing:
- High-throughput capacity: Parallel processing of dozens of live embryos.
- Precise spatial control: Generation of arbitrary, synthetic signaling patterns (e.g., Nodal patterns) with high resolution.
- Live imaging integration: Ability to monitor the outcomes of optogenetic perturbations in real-time.
Detailed Experimental Protocols
Protocol 1: Optogenetic Patterning of Nodal Signaling in Zebrafish Embryos
This protocol describes a pipeline for creating designer Nodal signaling patterns in live zebrafish embryos using improved Cry2/CIB1N-based optoNodal2 reagents and patterned illumination [6].
Diagram 1: Optogenetic Nodal Signaling Pathway
Materials and Reagents
Table 3: Key Research Reagent Solutions for Optogenetic Nodal Patterning
| Item | Function/Description | Example/Source |
|---|---|---|
| optoNodal2 Reagents | Improved optogenetic reagents (Cry2/CIB1N fusions) with minimal dark activity and enhanced kinetics for Nodal receptor activation. | [6] |
| Ultra-Widefield Microscope | Custom microscopy platform for parallel light patterning and imaging in up to 36 embryos. | [6] |
| Zebrafish Embryos | Wild-type or Nodal signaling mutant embryos. | Standard supplier |
| Low-Melt Agarose | For embedding and immobilizing embryos during imaging and patterning. | Standard supplier |
Step-by-Step Procedure
Sample Preparation:
- Inject zebrafish embryos at the one-cell stage with mRNA encoding the optoNodal2 constructs.
- Raise injected embryos in standard E3 embryo medium until the desired developmental stage (e.g., sphere stage, prior to gastrulation).
- For imaging and patterning, dechorionate the embryos and embed in low-melt agarose within a specialized sample carrier or imaging dish.
System Setup and Calibration:
- Mount the sample dish onto the stage of the ultra-widefield patterned illumination microscope.
- Using the system software, define the desired spatial pattern of blue light activation (e.g., a gradient, stripe, or spot) for the Nodal signal. The improved dynamic range of the optoNodal2 reagents allows for signaling levels that approach peak endogenous responses.
Light Patterning and Live Imaging:
- Apply the programmed light pattern to the embryos. The system can deliver this pattern to all 36 embryos in parallel.
- Simultaneously, use the live imaging capability of the system (e.g., with a far-red channel) to monitor downstream events, such as the nuclear translocation of a pSmad2 fluorescent biosensor or the expression of a target gene reporter (e.g., gsc or sox32).
Post-Processing and Analysis:
- Process the acquired images to quantify the spatial correlation between the delivered light pattern and the resulting biological response (e.g., gene expression domain, cell internalization movements).
- In Nodal signaling mutants, this patterned activation can be used to rescue characteristic developmental defects, such as controlled internalization of endodermal precursors [6].
Protocol 2: Multi-Scale Imaging of 3D Organoids from Live-Cell to Volume EM
This protocol enables uninterrupted correlation of live-cell light microscopy with nanometer-scale volume electron microscopy on the same 3D organoid, providing a continuum-resolution view of cellular processes [61].
Diagram 2: Multi-Scale 3D Organoid Imaging Workflow
Materials and Reagents
- High-Pressure Freezing (HPF) Carriers: 200-µm-deep gold-coated copper planchets.
- 3D Culture Matrix: Matrigel or other ECM hydrogels.
- Cell Lines: Mouse or human organoid lines (e.g., patient-derived colorectal cancer organoids).
- Culture Medium: Organoid-specific growth medium.
- Freeze-Substitution Medium: Containing 0.1% uranyl acetate in acetone for contrast while preserving fluorescence.
- Embedding Resin: Lowicryl HM20 or similar.
Step-by-Step Procedure
Culture in HPF Carriers:
- Pipette 1–2 µL of cell suspension mixed with Matrigel directly into the recess of an HPF carrier. This avoids the need for later transfer of fragile organoids.
- Place the carrier in a multi-well tissue culture dish supplied with medium, fixing it to a coverslip with a Matrigel droplet to prevent floating.
- Monitor organoid growth for up to 24 days using stereomicroscopy or live-cell confocal microscopy.
High-Pressure Freezing and Freeze-Substitution:
- At the desired time point, high-pressure freeze the entire HPF carrier containing the organoids to vitrify the sample, preserving its native ultrastructure.
- Transfer the frozen carrier to a freeze-substitution device. Process the sample in a cocktail containing 0.1% uranyl acetate in acetone to provide EM contrast while retaining fluorescence for later correlation.
Resin Embedding and Relocation:
- Infiltrate and embed the sample in Lowicryl HM20 resin.
- After polymerization, image the resin block with a confocal microscope to relocate the features of interest previously identified during live-cell imaging.
Targeted Volume Electron Microscopy:
- Use a two-photon laser to ablate landmarks on the resin block surface, guiding the subsequent FIB-SEM.
- Mount the block in a FIB-SEM and use the landmarks to locate the region of interest.
- Acquire a serial stack of images via iterative FIB ablation and SEM imaging. This generates a large volume of 3D ultrastructural information at nanometer resolution.
Data Integration and Analysis:
- Use trainable deep-learning automated image segmentation to annotate and quantitatively analyze subcellular structures (e.g., cell junctions, organelles) within the large EM datasets, linking ultrastructure to light microscope observations [61].
The challenges of delivering light efficiently deep within tissues and 3D cultures are significant but surmountable. By leveraging tailored technologies—such as light sheet microscopy for low-phototoxicity imaging, advanced optogenetic reagents with high dynamic range, ultra-widefield systems for high-throughput patterning, and integrated multi-scale workflows—researchers can overcome the barriers of scattering, phototoxicity, and poor resolution. The protocols outlined here provide a concrete roadmap for applying these solutions to cutting-edge research, enabling unprecedented precision in controlling and observing complex biological processes like gastrulation within physiologically relevant 3D environments.
The precise control of developmental signals with high resolution in space and time is fundamental to dissecting how embryonic cells decode morphogen information to make appropriate fate decisions. A significant challenge in employing optogenetic tools for such investigations is "leakiness"—unwanted baseline activity of the signaling pathway in the absence of the activating light stimulus. This undesired background activity can obscure the true morphogen signal, lead to erroneous cell fate decisions, and compromise the interpretation of experimental results. Within the context of controlling cell internalization during gastrulation, achieving a low-noise, tightly controlled system is paramount for establishing causal relationships between patterned Nodal signaling and subsequent morphogenetic movements. This Application Note details protocols and strategies for minimizing baseline leakiness, drawing on recent advances in optogenetic reagent engineering and experimental methodology.
Quantitative Analysis of Leakiness in Optogenetic Reagents
The performance of optogenetic tools can be quantified by their dynamic range, which is the ratio between the maximum light-induced signaling activity and the baseline activity in the dark. An ideal reagent exhibits negligible dark activity and a strong response upon illumination.
Table 1: Key Performance Metrics for Optogenetic Reagents Controlling Developmental Signaling Pathways
| Optogenetic System | Target Pathway | Organism | Reported Dark Activity | Light-Induced Activity | Dynamic Range | Primary Strategy for Reducing Leakiness |
|---|---|---|---|---|---|---|
| First-Generation optoNodal (LOV domain) [6] | Nodal | Zebrafish | Significant ("dark activity") [6] | Strong target gene expression [6] | Limited [6] | (Baseline for comparison) |
| optoNodal2 (Cry2/CIB1N) [6] | Nodal | Zebrafish | Eliminated ("eliminates dark activity") [6] | Improved, approaching endogenous levels [6] | Enhanced [6] | Receptor sequestration; Improved protein pair |
| optoRTK (LOV domain) [6] | Receptor Tyrosine Kinase | Cell Culture | Varies by design | High | High | Receptor sequestration [6] |
Table 2: Comparison of Optogenetic Protein Pairs and Their Properties
| Optogenetic Pair | Response Kinetics | Dark State Stability | Ease of Implementation | Suitability for Gastrulation Studies |
|---|---|---|---|---|
| LOV domains (e.g., Aureochrome 1) [6] | Slow dissociation (minutes) [6] | Can exhibit problematic dark activity [6] | Established | Limited by slow kinetics and leakiness |
| Cry2/CIB1N [6] | Fast ("improved response kinetics") [6] | High ("eliminates dark activity") [6] | Requires two-component expression | Excellent, used in optoNodal2 |
Experimental Protocols for Validating Tight Control
Protocol: Assessing Baseline Activity and Dynamic Range in Zebrafish Embryos
This protocol is designed to quantify the leakiness and light-induced efficacy of an optogenetic reagent, such as optoNodal2, in a live embryo context [6].
Key Materials:
- Genetically engineered zebrafish embryos expressing the optogenetic construct(s).
- Controlled illumination system capable of delivering precise blue light patterns (e.g., ultra-widefield microscopy platform) [6].
- Immunostaining reagents for phosphorylated Smad2 (pSmad2) and downstream target genes (e.g., gsc, sox32).
- Confocal or light-sheet microscope for high-resolution imaging.
Procedure:
- Embryo Preparation: Divide injected embryos into three groups:
- Experimental Group (Light): Embryos expressing the optogenetic reagent (e.g., optoNodal2).
- Dark Control Group: Embryos expressing the optogenetic reagent, kept in complete darkness or under minimal safe light.
- Wild-Type Control Group: Non-engineered embryos.
- Light Stimulation: At the desired developmental stage (e.g., shield stage), expose the Experimental Group to a uniform blue light pattern (e.g., 488 nm laser at a predetermined low intensity) for a set duration. Maintain Dark Controls in the dark.
- Fixation and Staining: At a predetermined time post-stimulation (e.g., 30-60 minutes), fix all embryo groups and perform immunostaining for pSmad2 to visualize immediate pathway activation and for downstream mesendodermal markers (e.g., gsc mRNA by in situ hybridization) to assess functional output.
- Image Acquisition and Quantification: Image all embryos under identical settings. Quantify the nuclear intensity of pSmad2 staining and the domain of expression of target genes in the relevant regions (e.g., margin).
- Data Analysis: Compare the staining intensity and domain size between the three groups. A successful, tight-control reagent will show pSmad2 and target gene expression in the Experimental Group similar to wild-type Nodal signaling, and no expression in the Dark Control Group, matching the wild-type negative control.
Protocol: Implementing a Sequestration Strategy to Minimize Leakiness
This protocol outlines the molecular engineering strategy used in the development of optoNodal2 to achieve minimal baseline activity [6].
Key Materials:
- DNA constructs for the Nodal type I receptor (e.g., Acvr1b) and type II receptor (e.g., Acvr2b).
- DNA for the light-sensitive heterodimerizing pair Cry2 and CIB1N.
- Molecular biology reagents for fusion protein construction (PCR, ligase).
- Localization tags (e.g., nuclear export signal, membrane anchor).
Procedure:
- Generate Receptor Fusions: Create fusion constructs where the intracellular domains of the Nodal type I receptor are fused to CIB1N, and the type II receptor is fused to Cry2.
- Engineer Cytosolic Sequestration: To further reduce the chance of spontaneous receptor interaction in the dark, modify the type II receptor (Cry2-fusion) by adding a strong nuclear export signal (NES) or removing its native transmembrane domain. This strategy forces the type II receptor to be sequestered in the cytosol, physically separating it from the membrane-bound type I receptor [6].
- Validate Subcellular Localization: Transiently express the individual constructs in a cell line (e.g., HEK293T) and confirm via live-cell imaging that the type I receptor is membrane-localized and the type II receptor is cytosolic.
- Test in a Functional Assay: Co-express the constructs in a cell-based reporter assay (e.g., a Smad-responsive luciferase reporter) to confirm minimal activity in the dark and strong, light-inducible activation.
- Generate Transgenic Organisms: Once validated in vitro, use the constructs to generate stable transgenic zebrafish lines for in vivo gastrulation studies.
Visualizing the Strategy for Tight Optogenetic Control
The following diagram, generated using Graphviz DOT language, illustrates the core molecular mechanism for achieving minimal leakiness in the improved optoNodal2 system.
Diagram Title: Mechanism of Leak-Free OptoNodal2 Control
The Scientist's Toolkit: Essential Reagents for Optogenetic Gastrulation Studies
Table 3: Research Reagent Solutions for Controlled Optogenetic Experiments
| Reagent / Tool | Function / Description | Justification for Use |
|---|---|---|
| optoNodal2 Reagents [6] | Cry2/CIB1N-fused Nodal receptors with cytosolic sequestration of the type II receptor. | The benchmark for low-dark-activity control of Nodal signaling; enables high-fidelity patterning. |
| Ultra-Widefield Patterned Illumination [6] | Microscope system for parallel light patterning in up to 36 embryos. | Enables high-throughput application of complex light patterns, essential for statistical power in gastrulation studies. |
| Phospho-Smad2 (pSmad2) Antibody | Marker for immediate, direct Nodal pathway activation. | Provides a quantitative readout of signaling activity independent of downstream transcription. |
| Light-Sheet Microscope [49] | Microscope that illuminates a single plane, reducing phototoxicity and enabling long-term live imaging. | Ideal for observing the dynamics of cell internalization in response to optogenetic stimulation during gastrulation. |
| Cry2/CIB1N Optogenetic Pair [6] | A blue-light-sensitive protein pair with fast kinetics and high dark-state stability. | A superior alternative to LOV domains for constructing new low-leakage optogenetic tools. |
The optical control of cell internalization during gastrulation represents a frontier in developmental biology, offering unprecedented spatiotemporal precision for probing fundamental morphogenetic processes. Optogenetic tools, particularly those based on Arabidopsis cryptochrome 2 (CRY2) and various Light-Oxygen-Voltage (LOV) domains, have emerged as powerful assets for manipulating signaling pathways that govern gastrulation. However, their effective application is hampered by tool-specific pitfalls—unwanted CRY2 aggregation and variable LOV domain kinetics—that can compromise experimental outcomes and data interpretation.
This Application Note addresses these critical challenges within the context of controlling cell internalization, a process fundamental to gastrulation across model organisms. We provide a mechanistic dissection of these pitfalls, quantitative comparisons of engineered solutions, detailed protocols for implementation, and strategic recommendations to empower researchers to select and optimize these molecular tools effectively.
Understanding and Controlling CRY2 Homo-oligomerization
The Molecular Basis of Uncontrolled CRY2 Clustering
The CRY2 photolyase homology region (PHR) exhibits both light-dependent homo-oligomerization and hetero-dimerization (e.g., with CIB1). While both properties are exploitable, a significant challenge arises when using CRY2-CIB1 for hetero-dimerization: unintended CRY2-CRY2 homo-oligomerization occurs simultaneously, leading to aberrant protein clustering and confounding experimental results [24].
Mechanistic studies have revealed that CRY2 interactions are governed by distinct interfaces:
- N-terminal charges are critical for CRY2-CIB1 hetero-dimerization
- C-terminal residues, particularly the electrostatic properties of positions 489 and 490, dictate homo-oligomerization propensity, with positive charges facilitating and negative charges inhibiting oligomerization [24]
Table 1: Engineered CRY2 Variants for Controlled Oligomerization
| Variant | Mutation/Feature | Oligomerization Property | Primary Application | Key Reference |
|---|---|---|---|---|
| CRY2(WT) | Wild-type (1-498) | Moderate baseline oligomerization | General use | [24] |
| CRY2olig | E490G | Enhanced, robust clustering | Strategies requiring strong self-association | [63] |
| CRY2high | Engineered C-terminal positive charges | Elevated oligomerization | Activation/sequestration requiring high dynamic range | [24] [23] |
| CRY2low | Engineered C-terminal negative charges | Suppressed oligomerization | CRY2-CIB1 applications to minimize interference | [24] [23] |
| CRY2low-tdTom | CRY2low fused to tandem dimeric Tomato | Sterically hindered oligomerization | High-specificity hetero-dimerization studies | [24] |
| CRY2clust | C-terminal 9-aa extension (e.g., residues 1-507) | Rapid, efficient clustering | Efficient recruitment and sequestration | [35] |
Practical Considerations for CRY2 Fusion Constructs
Beyond point mutations, the choice of fusion tags and their quaternary structure significantly impacts CRY2 clustering behavior. Systematic analysis reveals that fluorescent proteins (FPs) with dimeric (EYFP, Ypet) or tetrameric (DsRed) states enhance light-induced CRY2 clustering, whereas monomeric FPs produce more moderate effects [35]. The conjugation site (N- vs. C-terminal) also influences efficiency, with C-terminal fusions generally exhibiting higher clustering propensity [35].
For gastrulation studies aiming to control cell internalization—a process driven by actomyosin contraction and apical constriction—unintended protein clustering can disrupt delicate force-generation mechanisms. Therefore, selecting CRY2 variants with appropriate oligomerization properties is essential for precise experimental outcomes.
Managing LOV Domain Kinetics for Gastrulation Studies
Kinetic Diversity Among LOV Domains
LOV domains, another prominent class of blue-light photoreceptors, exhibit remarkable diversity in their photocycle kinetics, which profoundly impacts their utility in gastrulation research. These kinetics govern the temporal stability of the light-activated signaling state and must be matched to the biological process under investigation.
Table 2: Kinetic Properties of Representative LOV Domains
| LOV Domain Source | Photocycle Half-Life (Ï„) | Key Structural Features | Optogenetic Considerations | Reference |
|---|---|---|---|---|
| ZEITLUPE (ZTL) | ~1.4 hours | Unique dark-state conformation | Suitable for processes requiring minute-to-hour scale activation | [64] |
| FKF1 | ~62 hours | Characteristic E-F loop | Ideal for sustained signaling without constant illumination | [65] [64] |
| Aureochrome1 (VfAU1-LOV) | Reversible within seconds | Jα and A'α helices for signal transduction | Appropriate for rapid, reversible control | [23] [66] |
| Phototropin (AsLOV2) | ~70 seconds | C-terminal Jα helix | Balanced for second-to-minute scale interventions | [23] |
Environmental Impacts on LOV Function
The intracellular environment significantly influences LOV domain behavior. In-cell spectroscopy reveals that macromolecular crowding and specific cellular components can:
- Slow dark-state recovery compared to in vitro conditions [66]
- Suppress structural changes in specific secondary elements [66]
- After signal progression pathways from sensor to effector domains [66]
For gastrulation researchers, these findings underscore the importance of characterizing LOV tool behavior in relevant embryonic contexts rather than relying solely on in vitro data.
Experimental Protocols for Tool Validation and Application
Protocol: Validating CRY2 Variant Performance in Gastrulation Studies
This protocol assesses CRY2 oligomerization propensity in live cells, with particular relevance for gastrulation research.
Materials:
- Mammalian expression vectors encoding CRY2 variants (CRY2wt, CRY2olig, CRY2low, etc.)
- Fluorescent protein tags (e.g., mCherry, EYFP)
- COS-7 or HEK293 cell lines
- Confocal microscopy system with 488nm laser capability
- Image analysis software (e.g., ImageJ, MetaMorph)
Procedure:
- Transfection: Plate cells on glass-bottom dishes and transfect with CRY2-FP constructs using standard methods.
- Image Acquisition:
- Maintain cells in darkness for 24h post-transfection
- Acquire pre-illumination images using low-intensity laser light
- Apply blue light stimulation (e.g., 488nm, 1.27 mW/cm²) with pulsed or continuous illumination
- Capture time-lapse images every 10s for 5-10min
- Quantitative Analysis:
- Calculate clustering ratio: (fluorescence in clusters)/(total cellular fluorescence)
- Determine clustering kinetics: time to half-maximal clustering (tâ‚/â‚‚)
- Assess reversibility: monitor cluster dissipation over 30-60min post-illumination
- Gastrulation-Relevant Functional Test:
- Fuse CRY2 variants to cytoskeletal regulators (e.g., RhoGEF, actin-nucleating proteins)
- Assess localization and clustering in response to illumination
- Evaluate functional consequences on cell morphology and movement
Expected Outcomes: CRY2olig and CRY2high should show rapid, robust clustering (70-90% of cytosolic protein in clusters within seconds-minutes). CRY2low and CRY2low-tdTom should exhibit minimal clustering (<10-20% of protein) under identical conditions [24] [63].
Protocol: Characterizing LOV Domain Kinetics in Live Embryos
This protocol measures the kinetic behavior of LOV domains in developing embryos, providing critical data for tool selection in gastrulation studies.
Materials:
- LOV domain constructs (ZTL, FKF1, VfAU1-LOV, etc.) fused to fluorescent reporters
- Model organism embryos (Xenopus, C. elegans, zebrafish)
- Blue LED illumination system with calibrated intensity
- Fluorescence microscope with environmental control
- Analysis software for fluorescence quantification
Procedure:
- Sample Preparation:
- Inject in vitro transcribed mRNA encoding LOV-FP constructs into 1-4 cell stage embryos
- Culture embryos to desired developmental stage (e.g., gastrula)
- Dark-State Fluorescence Measurement:
- Maintain embryos in darkness prior to imaging
- Acquire baseline fluorescence images with minimal exposure
- Activation and Recovery Kinetics:
- Apply saturating blue light pulse (e.g., 470nm, 1.7-2.5 μW/mm² for 30s)
- Immediately monitor fluorescence changes (e.g., decrease in FMN fluorescence)
- Continue time-lapse imaging in darkness to track recovery
- Fit recovery curve to exponential function to determine half-life
- Functional Validation in Gastrulation Context:
- Target LOV domains to specific embryonic tissues (e.g., dorsal mesoderm)
- Assess ability to control cell internalization via light-activated constructs
- Correlate kinetic parameters with functional efficacy
Expected Outcomes: FKF1-LOV should exhibit prolonged signaling state (Ï„ ~62h), ZTL-LOV intermediate kinetics (Ï„ ~1.4h), and Aureochrome1-LOV faster recovery (minutes) [65] [64]. These kinetics should align with the temporal requirements of the gastrulation process being studied.
Strategic Implementation for Gastrulation Research
Matching Tool Properties to Biological Questions
The selection of optogenetic tools must be guided by the specific requirements of the gastrulation process under investigation:
For rapid, reversible control of cytoskeletal dynamics during apical constriction:
- Recommended: Aureochrome1-LOV or iLID systems with fast kinetics
- Rationale: These processes occur on second-to-minute timescales
For sustained modulation of gene expression in the dorsal organizer:
- Recommended: FKF1-LOV or CRY2olig with prolonged signaling states
- Rationale: Transcriptional changes require persistent activation
For precise spatial control of cell internalization without collateral clustering:
- Recommended: CRY2low-tdTom with CIB1 for hetero-dimerization
- Rationale: Minimizes interference from homo-oligomerization
Diagram: Strategic Selection of Optogenetic Tools
Essential Research Reagent Solutions
Table 3: Key Reagents for Optogenetic Gastrulation Studies
| Reagent Category | Specific Examples | Function/Application | Source/Reference |
|---|---|---|---|
| CRY2 Variants | CRY2wt, CRY2olig (E490G), CRY2high, CRY2low | Core optogenetic actuators for light-controlled dimerization/oligomerization | [24] [63] |
| LOV Domains | FKF1-LOV, ZTL-LOV, Aureochrome1-LOV (VfAU1-LOV), AsLOV2 | Blue-light receptors with varied kinetics for different temporal requirements | [23] [65] [64] |
| Interaction Partners | CIB1 (for CRY2), SspB (for iLID), GI (for ZTL/FKF1) | Protein partners for hetero-dimerization systems | [24] [23] |
| Fluorescent Tags | mCherry, EYFP, Ypet, DsRed, tdTomato | Visualization and clustering modulation | [35] |
| Actuation Equipment | Blue LED systems (470nm), confocal microscopy with 488nm laser | Precise light delivery for optogenetic activation | [23] |
Successful optogenetic control of cell internalization during gastrulation demands careful consideration of tool-specific pitfalls. CRY2 variants with engineered oligomerization properties (CRY2olig, CRY2high, CRY2low) provide a toolkit for balancing clustering propensity with experimental requirements. Similarly, LOV domains with diverse photocycle kinetics (from rapid Aureochrome1 to slow FKF1) enable temporal matching to specific gastrulation processes. By applying the validation protocols and strategic selection framework outlined here, researchers can overcome these technical challenges and harness the full potential of optogenetics to dissect the complex mechanisms governing gastrulation.
Benchmarking Optogenetics: Validation Against Established Methods and Future Potential
A fundamental challenge in developmental biology is linking the perturbation of a signaling pathway to the resulting morphological outcomes. The emergence of optogenetics provides an unprecedented toolset for controlling morphogen signals with high spatiotemporal precision in live embryos [6]. This application note details protocols for using next-generation optogenetic tools to control Nodal and Rho signaling, two pathways critical for gastrulation, and provides a framework for quantitatively correlating the induced phenotypes with endogenous developmental processes. By creating defined, light-based signaling patterns, researchers can move beyond observation to actively test hypotheses about how embryos decode signaling information to guide cell internalization and tissue shaping [6] [17].
The Scientist's Toolkit: Essential Research Reagents
The following table catalogs key reagents and tools essential for implementing optogenetic control in developmental studies.
Table 1: Key Research Reagent Solutions for Optogenetic Perturbation Studies
| Reagent/Tool Name | System | Function and Key Features |
|---|---|---|
| OptoNodal2 [6] | Zebrafish | An improved optogenetic reagent using Cry2/CIB1N heterodimerization to control Nodal receptors. It eliminates dark activity and offers improved kinetics for precise spatial patterning of Nodal signaling. |
| Endogenous OptoRhoGEFs (OptoGEF2, OptoCysts) [17] | Drosophila | RhoGEFs (RhoGEF2, Cysts) endogenously tagged with the iLID/SspB system. Enables light-dependent control of endogenous Rho signaling with minimal expression-level variability and high embryonic viability. |
| Ultra-Widefield Patterned Illumination Platform [6] | Zebrafish | A custom microscopy system for parallel light patterning and live imaging in up to 36 embryos simultaneously. Enables high-throughput generation of synthetic signaling patterns. |
| Two-Photon (2P) Activation [17] | Drosophila | An optical strategy for achieving subcellular recruitment of optogenetic reagents like iLID/SspB, providing high z-axis specificity for 3D-patterned perturbations. |
| Membrane-Targeted iLID Construct (e.g., iLID-CaaX) [17] | Drosophila | A crucial component of the iLID/SspB system. The CaaX motif localizes the protein to the plasma membrane, serving as the anchor for recruiting SspB-tagged effector proteins. |
Experimental Protocols for Optogenetic Control
Protocol 1: Patterning Nodal Signaling in Zebrafish Embryos
This protocol describes a pipeline for generating synthetic Nodal signaling patterns in zebrafish embryos using the optoNodal2 system [6].
- Objective: To spatially control Nodal signaling activity and downstream gene expression, and to quantify the resulting cell internalization behaviors during gastrulation.
- Materials:
- Zebrafish embryos injected with mRNA for optoNodal2 components (CIB1N-fused type I receptor, cytosolic-Cry2-fused type II receptor).
- Ultra-widefield patterned illumination microscope.
- Standard reagents for zebrafish embryo maintenance and mounting.
- Procedure:
- Embryo Preparation: Inject one-cell stage zebrafish embryos with mRNAs encoding the optoNodal2 constructs. Raise embryos in the dark to prevent unintended activation.
- Mounting: At the desired developmental stage (e.g., sphere or shield stage), mount the embryos in agarose for live imaging and light patterning.
- Light Patterning: Using the custom illumination platform, project user-defined geometric patterns of blue light (e.g., 488 nm) onto the embryos. The pattern can be a gradient, stripes, or a confined spot, depending on the experimental question.
- Validation and Imaging:
- Signaling Activity: Fix embryos at specific time points and immunostain for phosphorylated Smad2 (pSmad2) to visualize the spatial pattern of Nodal signaling activity.
- Gene Expression: Perform in situ hybridization for early Nodal target genes (e.g., gsc, ntl) to assess downstream transcriptional responses.
- Cell Behavior: Use time-lapse imaging of live embryos to track the internalization movements of endodermal precursors in response to the patterned light signal.
- Validation & Data Analysis:
- Correlate the light pattern with the resulting pSmad2 immunostaining pattern to quantify the fidelity and dynamic range of signaling induction.
- Quantify the extent of cell internalization by measuring the change in position of cells within the patterned region over time.
Protocol 2: Inducing Epithelial Furrowing via Endogenous RhoGEFs inDrosophila
This protocol outlines the use of endogenously tagged OptoRhoGEFs to induce and quantify epithelial furrowing in Drosophila embryos [17].
- Objective: To achieve quantitative, 3D-patterned perturbations of cell contractility and measure the dose-dependent relationship between RhoGEF recruitment and tissue furrow depth/orientation.
- Materials:
- Drosophila embryos from the OptoGEF2 or OptoCysts knock-in lines.
- Embryos expressing a membrane-targeted iLID construct (e.g., UASp>mCherry-iLID-CaaX) in the desired tissue.
- Two-photon microscope for spatially confined activation.
- Confocal microscope for live imaging of fluorescently tagged cytoskeletal proteins (e.g., myosin).
- Procedure:
- Embryo Collection and Preparation: Collect embryos from the appropriate cross and age them to the blastoderm or early gastrulation stage.
- Two-Photon Activation: Use a two-photon laser (e.g., ~920 nm) to scan a defined 3D pattern on the embryo surface, recruiting the endogenous RhoGEFs to the plasma membrane in the illuminated voxels.
- Live Imaging: Simultaneously or immediately after activation, perform time-lapse confocal imaging to capture the dynamics of tissue deformation and the localization of fluorescent reporters for actomyosin.
- Validation & Data Analysis:
- Dose-Response: Quantify the recruitment level of the RhoGEF (via RFP fluorescence) and correlate it with the resulting furrow depth. This reveals the dose-dependence of the morphogenetic output.
- Mechanical Context: Disrupt the basal actomyosin network (e.g., via scraps RNAi) and repeat the experiment to test its role in constraining and orienting the light-induced furrow [17].
The quantitative application of these optogenetic tools allows for precise measurement of input-output relationships in development. The following table summarizes key quantitative findings from the referenced studies.
Table 2: Summary of Quantitative Data from Optogenetic Perturbations
| System / Measured Parameter | Experimental Condition | Quantitative Outcome | Biological Implication |
|---|---|---|---|
| OptoNodal2 (Zebrafish) [6] | Dynamic range of signaling | Elimination of dark activity; high light-induced signaling. | Enables creation of precise, synthetic Nodal patterns without background noise. |
| OptoGEF2 (Drosophila) [17] | Embryonic viability (at 23°C in dark) | 0.87 (vs. 0.83 in wild-type). | Endogenous tagging preserves healthy development, providing a physiologically relevant context. |
| RhoGEF2(DHPH)-Cry2 (Drosophila) [17] | Embryonic viability (at 23°C in dark) | 0.38. | Highlights the deleterious effects of transgenic overexpression. |
| Two-Photon Activation [17] | Z-axis specificity of recruitment | 4.2 ± 0.3 µm. | Enables high-precision 3D patterning of contractility within a tissue. |
| OptoGEF2 Activity [17] | RhoGEF recruitment level vs. furrow depth | A direct dose-dependent relationship was observed. | Tissue morphology can be quantitatively shaped by modulating the level of RhoGEF activity. |
Signaling Pathway and Experimental Workflow Diagrams
The following diagrams illustrate the core molecular mechanisms and experimental workflows described in this application note.
Diagram 1: Molecular logic of the optogenetic tools for controlling Nodal and Rho signaling.
Diagram 2: Generalized experimental workflow for optogenetic validation of developmental processes.
Spatiotemporal control represents a paradigm shift in biological research and therapeutic development, enabling scientists to manipulate cellular and molecular processes with unprecedented specificity in both time and space. This approach is particularly critical in developmental biology, where processes like gastrulation involve highly coordinated cell movements and fate decisions. By leveraging tools that can be switched "on" and "off" with precision, researchers can now dissect complex mechanisms such as cell internalization during germ layer formation without permanently disrupting native physiology. The unique advantages of precision and reversibility offered by these methods minimize off-target effects and allow for dynamic intervention that mirrors natural biological processes, providing more accurate models for studying development and disease.
Core Methodologies for Spatiotemporal Control
Optogenetic Control
Optogenetics utilizes light-sensitive proteins to control biological function with exceptional spatiotemporal precision. The LightR system exemplifies this approach, employing a engineered blue-light-activated allosteric switch derived from two tandem Vivid (VVD) photoreceptor domains from Neurospora crassa [67].
Mechanism of Action: In the dark state, the LightR domain remains open, distorting the catalytic domain of the fused protein and maintaining it in an inactive state. Upon exposure to blue light (465 nm), the LightR domain closes, restoring the native protein structure and enzymatic activity [67]. This reversible control enables precise temporal regulation of protein function.
Engineering Considerations: Successful implementation requires strategic insertion of LightR into flexible loop regions of the target protein that are structurally coupled to key catalytic elements but are not existing binding sites. The insertion site must allow for LightR-induced conformational changes without disrupting essential protein functions or interactions [67].
Photo-Responsive Molecular Engineering
An alternative to genetic encoding involves chemical modification of cell surfaces with photo-switchable components. This method combines metabolic glycan labeling with bio-orthogonal click chemistry to install host molecules on cell membranes, enabling light-controlled reversible interactions [68].
Implementation Workflow: Cells are treated with peracetylated N-azidoacetylgalactosamine (Ac4GalNAz) to incorporate azide tags into surface glycoconjugates. Alkynyl-PEG-β-cyclodextrin is then conjugated via copper-catalyzed azide-alkyne cycloaddition. The resulting β-cyclodextrin-modified surfaces can interact with azobenzene-labeled molecules, with binding affinity that can be reversibly controlled by light due to the isomerization properties of azobenzene [68].
Advantages: This approach provides rapid, reversible control without genetic modification, making it applicable to primary cells and complex co-culture systems. The covalent nature of the surface modification offers greater stability than lipid-based insertion methods [68].
Table 1: Comparison of Spatiotemporal Control Methodologies
| Method | Spatial Precision | Temporal Resolution | Reversibility | Key Applications |
|---|---|---|---|---|
| Optogenetics (LightR) | Subcellular | Seconds to minutes | High | Protein activity control, Signaling pathway manipulation [67] |
| Photo-responsive Surface Engineering | Cellular | Minutes | High | Cell-cell interactions, Tissue assembly [68] |
| Chemical Inducers | Tissue to organism level | Hours to days | Moderate to low | Gene expression control, Bacterial therapy [69] |
| Magnetic Manipulation | Millimeter to centimeter | Minutes | Variable | Bacterial drug delivery, Cellular microrobots [69] |
Experimental Protocols
Protocol: Implementing LightR for Spatiotemporal Control of Kinase Activity
This protocol details the application of LightR for controlling Src tyrosine kinase activity, with principles applicable to various enzymes [67].
Materials Required:
- LightR-Src construct (generated by inserting LightR into Src kinase domain)
- Appropriate cell line for transfection (e.g., HEK293, fibroblasts)
- Blue light source (465 nm) with controlled intensity and timing
- Culture medium and standard tissue culture reagents
- Immunoblotting supplies for phosphorylation analysis
Procedure:
- Cell Seeding and Transfection: Seed cells onto appropriate culture vessels and transfect with LightR-Src construct using preferred method. Include controls: constitutive active Src and kinase-dead Src.
- Starvation and Synchronization: Serum-starve cells for 4-6 hours prior to stimulation to reduce basal kinase activity.
- Light Stimulation: Expose experimental groups to blue light (465 nm) using calibrated illumination system. Maintain control groups in darkness.
- Activation Kinetics: For continuous activation, apply sustained illumination. For pulsed activation, program light source for specific intervals (e.g., 30 seconds on, 2 minutes off).
- Sample Collection and Analysis: Harvest cells at predetermined time points. Analyze Src activation status via immunoblotting for autophosphorylation (Tyr419) or phosphorylation of downstream substrates.
Troubleshooting:
- Low dynamic range: Optimize LightR insertion site or try different linker lengths
- High background activity: Ensure proper darkness maintenance for control groups
- Poor expression: Verify construct design and transfection efficiency
Protocol: Light-Controlled Reversible Cell-Cell Interactions
This protocol describes engineering photo-responsive surfaces for manipulating cell assemblies, applicable to studying contact-dependent signaling during gastrulation [68].
Materials Required:
- Ac4GalNAz (metabolic labeling precursor)
- Alkynyl-PEG-β-cyclodextrin (for click chemistry conjugation)
- Azobenzene-modified aptamers or adhesion molecules
- Copper(I) catalyst (TBTA, CuSOâ‚„, sodium ascorbate)
- Appropriate cell culture media and reagents
Procedure:
- Metabolic Labeling: Culture cells with Ac4GalNAz (50 μM final concentration) for 72 hours to incorporate azide groups onto surface glycans.
- β-Cyclodextrin Conjugation: Wash labeled cells and incubate with alkynyl-PEG-β-cyclodextrin (100 μM) in presence of copper(I) catalyst for 1 hour at room temperature.
- Surface Validation: Confirm β-cyclodextrin installation using azobenzene-DNA-FAM probe and flow cytometry or fluorescence microscopy.
- Assembly Induction: Incubate β-cyclodextrin-modified cells with azobenzene-functionalized partner cells or substrates (trans-azobenzene for binding).
- Light-Controlled Reversal: Expose assembled structures to UV light (365 nm) to isomerize azobenzene to cis-form, disrupting host-guest interactions and disassembling structures.
- Reassembly: To reform assemblies, expose to visible light to revert azobenzene to trans-isomer.
Applications: This system enables reversible manipulation of cell assemblies for studying contact-dependent signaling events relevant to germ layer formation and cell migration during gastrulation.
Visualization of Spatiotemporal Control Systems
LightR Optogenetic System Mechanism
Diagram 1: Mechanism of LightR optogenetic control system showing reversible conformational changes.
Photo-Responsive Cell Surface Engineering
Diagram 2: Photo-responsive cell surface engineering for reversible cell-cell interactions.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents for Spatiotemporal Control Experiments
| Reagent/Category | Function | Example Applications | Key Considerations |
|---|---|---|---|
| Light-Sensitive Domains (VVD, LightR, EL222) | Protein allosteric regulation | Kinase control, Transcription factor activation | Requires optimization of insertion site; leakage in dark state should be minimized [67] |
| Metabolic Labeling Agents (Ac4GalNAz) | Incorporation of bio-orthogonal handles | Cell surface engineering, Targeted delivery | Cell-type dependent efficiency; requires 48-72 hour incubation [68] |
| Bio-orthogonal Conjugation Partners (Alkynyl-PEG-β-cyclodextrin) | Covalent surface modification | Host-guest systems, Molecular recognition | PEG spacer enhances accessibility; copper catalysts may require optimization [68] |
| Photo-switchable Ligands (Azobenzene derivatives) | Light-controlled molecular recognition | Reversible assembly, Drug release | Quantum yield of isomerization; photostability over cycles [68] |
| Upconversion Nanoparticles | NIR to visible light conversion | Deep tissue optogenetics, In vivo applications | Composition affects conversion efficiency; potential cytotoxicity [69] |
Application Notes for Gastrulation Research
Contextualizing Spatiotemporal Control in Gastrulation Studies
Gastrulation involves precisely coordinated cell movements including internalization, migration, and fate specification, making it an ideal process for spatiotemporal control approaches. Recent spatial transcriptomic atlases of mouse gastrulation have revealed intricate gene expression patterns across anterior-posterior and dorsal-ventral axes, providing a foundation for targeted interventions [70]. The precision of optogenetic tools enables researchers to manipulate specific signaling pathways in defined cell populations at precise developmental timepoints, overcoming limitations of traditional genetic approaches that lack temporal control.
Practical Implementation Considerations
When applying spatiotemporal control systems to gastrulation research, several factors require careful consideration. For light-based systems, penetration depth can limit applications in thicker embryonic tissues. Strategies to address this limitation include:
- Using upconversion nanoparticles that transform deep-penetrating near-infrared light to visible wavelengths [69]
- Implementing two-photon excitation for improved tissue penetration
- Employing waveguide systems for targeted internal illumination
For chemical control systems, diffusion kinetics and metabolic stability must be optimized to achieve rapid and reversible effects in developing embryos. The development of pH-sensitive imaging probes like the BLINK system demonstrates how microenvironment-specific activation can provide additional spatial targeting precision [71].
Integration with Advanced Model Systems
Spatiotemporal control methods are particularly powerful when combined with emerging gastrulation models such as gastruloids. The ability to project in vitro models onto in vivo spatial atlases [70] creates opportunities to validate findings across experimental systems. Synthetic gene circuits like synNotch provide modular platforms for engineering custom cell-cell communication systems that can be controlled with spatiotemporal precision [72]. These approaches enable researchers to test specific hypotheses about patterning mechanisms by manipulating signaling events with defined timing and location, then observing consequent changes in morphogenetic outcomes.
Future Perspectives
The field of spatiotemporal control continues to evolve with emerging technologies offering enhanced precision and minimal invasiveness. Advances in CRISPR/Cas9 regulation through optogenetic and chemical methods provide powerful tools for spatial-temporal genomic manipulation [73]. Multimodal approaches that combine multiple control strategies (optical, magnetic, acoustic) will enable increasingly sophisticated perturbations of developmental systems. As these technologies mature, they will provide deeper insights into the dynamic processes shaping embryonic development and offer new therapeutic strategies for developmental disorders.
The study of gastrulation, the critical developmental stage where a simple sheet of cells forms the three primary germ layers, has been revolutionized by the emergence of advanced engineered model systems and precision control tools. By recreating and manipulating these fundamental processes in vitro, researchers have moved beyond basic science into new therapeutic frontiers. The integration of synthetic biology, microfabrication technologies, and optogenetic control now enables unprecedented dissection of how cells internalize, organize, and commit to specific fates. These approaches provide a powerful framework for regenerative medicine by revealing how to reconstruct developmental pathways for tissue engineering, while simultaneously offering platforms for modeling human diseases that originate from errors in early embryonic patterning. This application note explores how technologies originally developed to study gastrulation—particularly light-based control of cell internalization—are being leveraged to build human disease models and engineer tissues with clinical potential.
Key Experimental Models and Their Applications
Engineered Embryonic Model Systems
Recent advances in stem cell biology have enabled the development of sophisticated in vitro models that recapitulate specific stages of embryonic development, offering ethically accessible and highly controllable platforms for research and therapeutic exploration.
Table 1: Engineered Embryonic Model Systems and Their Applications
| Model Type | Key Components | Developmental Stage Captured | Potential Applications |
|---|---|---|---|
| Blastoids [74] [7] | Naive stem cells (hESCs/iPSCs), Trophoblast stem cells (TSCs) | Pre-implantation blastocyst (5-7 days post-fertilization) | Studying infertility, implantation failure, contraceptive testing, early developmental defects |
| Gastruloids [74] [75] | Pluripotent stem cells (ESCs), Micropatterned substrates | Gastrulation (14+ days post-fertilization) | Modeling body plan organization, germ layer specification, teratogenicity testing, axial patterning disorders |
| ETiX Embryoids [75] | ES cells, TS cells, iXEN cells | Neurulation and early organogenesis (up to day 8.5 post-fertilization in mouse models) | Studying neural tube defects, cardiac development, organogenesis disorders, primordial germ cell formation |
| Somitoids [74] | Pluripotent stem cells, Patterned morphogen signals | Somitogenesis and axial elongation | Modeling segmentation disorders, vertebral defects, neuromuscular conditions, tissue-scale patterning |
Quantitative Outcomes of Embryo Models
The utility of these engineered systems in disease modeling and regenerative medicine is demonstrated through their ability to recapitulate key developmental milestones and morphological structures with high fidelity to natural embryogenesis.
Table 2: Developmental Outcomes in Advanced Embryo Models
| Model System | Key Developmental Structures Formed | Developmental Timeline | Efficiency of Development |
|---|---|---|---|
| ETiX Embryoids [75] | Headfolds with forebrain/midbrain regions, beating heart-like structure, neural tube, somites, gut tube, primordial germ cells | Up to day 8.5 post-fertilization equivalent | 10-15% of initial aggregates form well-organized structures; >70% transition efficiency between developmental stages |
| Blastoids [74] | Blastocoel cavity, pluripotent epiblast (OCT4+), trophectoderm (GATA3+), primitive endoderm (SOX17+) | 7 days to form blastocyst-like structures; culture up to 21 days | Over 80% efficiency from hiPSC/hESC populations; demonstrates implantation capacity |
| Gastruloids [74] | Trilaminar germ layer organization, primitive streak-like patterning, axial organization | 5-7 days to establish gastrulation-like patterning | Highly reproducible across cell lines; responsive to morphogen perturbation |
Experimental Protocols for Light-Controlled Morphogenesis
Optogenetic Control of Nodal Signaling in Zebrafish Embryos
The ability to precisely control morphogen signaling with light provides unprecedented spatial and temporal resolution for studying cell internalization mechanisms during gastrulation and their applications.
Protocol: Optogenetic Patterning of Nodal Signaling for Controlled Cell Internalization [6]
Background: Nodal is a TGF-β family morphogen that patterns the mesendoderm and guides cell internalization during gastrulation. This protocol enables spatial and temporal control of Nodal signaling using light, allowing precise manipulation of gastrulation movements.
Materials & Reagents:
- OptoNodal2 reagents: Type I (acvr1b) and Type II (acvr2b) Nodal receptors fused to Cry2/CIB1N heterodimerizing pair
- Zebrafish embryos: Wild-type or Nodal signaling mutants (sqt, cyc)
- Ultra-widefield microscopy platform: Custom system for parallel light patterning in up to 36 embryos
- Blue light source: 488nm laser with digital micromirror device for spatial patterning
Procedure:
- Microinjection: Inject optoNodal2 mRNA into 1-cell stage zebrafish embryos.
- Embryo mounting: Orient and mount dechorionated embryos in agarose wells for controlled light exposure.
- Light patterning program: Design spatial illumination patterns using custom software to define regions of Nodal activation.
- Activation protocol: Expose embryos to patterned blue light (488nm) beginning at shield stage (6 hours post-fertilization).
- Typical parameters: 5-30μW/mm² intensity, 1-10 minute cycles
- Live imaging: Monitor pSmad2 nuclear translocation (signaling readout) and cell internalization movements via time-lapse microscopy.
- Fixation and staining: Process embryos for in situ hybridization of Nodal target genes (sox32, gsc, ntl) or immunofluorescence.
Applications:
- Controlled internalization of endodermal precursors
- Rescue of endoderm and mesoderm patterning defects in Nodal mutants
- Spatial control of cell motility and adhesivity gradients during gastrulation
- Testing theoretical models of morphogen-mediated patterning
Generation of ETiX Embryoids for Organogenesis Studies
Protocol: Assembling Complete Embryo Models for Organogenesis and Disease Modeling [75]
Background: ETiX embryoids combine embryonic stem cells with extraembryonic stem cells to recapitulate development through neurulation and early organogenesis, providing a scalable platform for studying human development and disease.
Materials & Reagents:
- Mouse ES cells: Conventional embryonic stem cells
- TS cells: Trophoblast stem cells derived from extraembryonic ectoderm
- iXEN cells: Induced extraembryonic endoderm stem cells
- AggreWell plates: For controlled aggregation of stem cell populations
- Rotatory culture system: For extended embryo culture
Procedure:
- Cell preparation: Culture ES, TS, and iXEN cells separately in appropriate maintenance media.
- Aggregation: Combine the three cell types in AggreWell plates at optimized ratios (typically 10:5:1 ES:TS:iXEN).
- Day 0-4 culture: Maintain aggregates in suspension culture to permit self-organization and cavitation.
- Selection: Identify and select embryoids with correct morphology (proamniotic cavity, migrated AVE, gastrulating).
- Day 4-7 culture: Transfer selected structures to glucose-supplemented medium in stationary culture.
- Day 7-8 culture: Transfer to rotating culture bottles for final development.
- Analysis: Fix for single-cell RNA sequencing, immunofluorescence, or live imaging.
Key Quality Control Checkpoints:
- Day 4: Presence of cavitated epithelial compartments enveloped by VE-like layer
- Day 5: Formation of proamniotic cavity, migrated AVE, active gastrulation
- Day 7: Anterior-posterior axis establishment, neural fold formation
- Day 8: Brain region specification, heart beating, somite formation, blood island development
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for Embryonic Engineering
| Reagent/Cell Type | Function | Application Examples |
|---|---|---|
| Naive Human Stem Cells [74] | Foundational cells with developmental plasticity | Blastoid formation, pre-implantation modeling, lineage specification studies |
| Trophoblast Stem Cells (TSCs) [74] [75] | Contribute extraembryonic tissues | Modeling embryo-maternal interactions, placental development, implantation |
| Induced XEN cells (iXEN) [75] | Form extraembryonic endoderm lineages | Supporting anterior-posterior patterning, yolk sac development in embryoids |
| OptoNodal2 Reagents [6] | Light-controllable Nodal signaling components | Spatiotemporal control of mesendoderm patterning, cell internalization studies |
| AggreWell Plates [74] [75] | Microwell platforms for controlled cell aggregation | Standardized embryoid formation, high-throughput model generation |
| Micropatterned Substrates [74] | Defined adhesive regions controlling cell geometry | Studying symmetry breaking, germ layer specification, neural tube folding |
Signaling Pathways in Gastrulation and Morphogenesis
The molecular pathways controlling gastrulation represent potential therapeutic targets for congenital disorders and provide engineering principles for tissue regeneration.
Key Pathway Components and Manipulation Points:
Nodal Signaling Control Points [6]:
- Optogenetic intervention: Light-controlled receptor dimerization enables spatial patterning
- Downstream readouts: pSmad2 nuclear translocation, target gene expression (sox32, gsc)
- Biological outcomes: Mesendoderm patterning, germ layer specification, cell internalization
Actomyosin Contractility Pathway [9]:
- Polarity regulation: PAR proteins control apical myosin localization (NMY-2)
- Force generation: Phosphorylated myosin drives apical constriction
- Evolutionary variation: Diverse upstream regulators converge on conserved cytoskeletal machinery
Integration Points for Engineering:
The engineering of gastrulation through light-controlled systems and advanced embryo models has created powerful platforms that extend far beyond developmental biology. These approaches enable precise dissection of disease mechanisms underlying congenital disorders, provide human-relevant models for drug screening, and establish engineering principles for tissue regeneration. As these technologies mature, they promise to bridge fundamental developmental insights with clinical applications, particularly for disorders originating from errors in early embryonic patterning, cell internalization, and tissue organization. The integration of increasingly sophisticated optogenetic tools with multi-lineage embryo models represents a promising frontier for regenerative medicine and disease modeling.
Gastrulation, occurring approximately two weeks post-fertilization in humans, represents a pivotal phase in embryonic development where a flat sheet of cells undergoes dramatic transformation to establish the foundational body plan, including the head/tail, ventral/dorsal, and right/left axes [29]. While genetic and biochemical signals have long been recognized as directors of this process, recent groundbreaking research has illuminated the fundamental role of mechanical forces in orchestrating gastrulation events [76]. The emergence of optogenetic technologies now provides unprecedented precision in manipulating these physical forces within developing embryos, opening new avenues for fundamental discovery and potential therapeutic innovation [76]. This application note details how insights from light-controlled embryology can bridge the gap between basic mechanical principles and clinical applications, with a specific focus on controlling cell internalization during gastrulation.
Table 1: Summary of Quantitative Findings from Gastrulation Studies
| Study Model | Key Manipulation | Measured Outcome | Quantitative Results | Biological Significance |
|---|---|---|---|---|
| Human Embryo (Light-Controlled) [76] | Increased mechanical force on one side | Embryonic curvature | Embryo bent toward opposite side | Mechanical force directly influences embryonic shaping |
| Human Embryo (Light-Controlled) [76] | Decreased mechanical force on one side | Embryonic curvature | Embryo bent toward the same side | Balanced forces are required for proper axis formation |
| Human Embryo (Light-Controlled) [76] | Varied mechanical force | Embryo dimensions | Length increased, width decreased with added force | Force patterns directly regulate axial extension |
| Drosophila Embryo [77] | Natural gastrulation | Apical constriction rate | Maximum 40% surface reduction at t≈2 min | Distinct temporal phases in gastrulation mechanics |
| Mouse ESCs & Embryonic Cultures [78] | Extracellular Stx4 inhibition | Brachyury expression | Increased expression | Specific molecular trigger for gastrulation initiation |
Table 2: Cellular & Molecular Responses to Mechanical Cues
| Parameter Assessed | Experimental System | Observed Effect | Downstream Consequences |
|---|---|---|---|
| Cell Differentiation [76] | Human Embryo (Light-Controlled) | Altered differentiation patterns | Mechanical environment influences cell fate decisions |
| Gene Expression [76] | Human Embryo (Light-Controlled) | Specific genes up/down-regulated | Mechanical forces directly influence genetic programs |
| Tissue Stiffness [77] | Drosophila Embryo | Lateral cells stiff, dorsal cells soft | Differential mechanical properties guide morphogenesis |
| FAK/AKT/PI3K Signaling [78] | Mouse ESCs & Embryonic Cultures | Pathway deactivation | Altered P-cadherin expression and brachyury induction |
| Rho/ROCK Signaling [78] | Mouse ESCs & Embryonic Cultures | Pathway activation | Morphological changes in embryonic stem cells |
Experimental Protocols
Protocol 1: Optogenetic Manipulation of Mechanical Forces in Embryos
Purpose: To precisely control mechanical forces in early human embryos using light-sensitive molecules to investigate their role during gastrulation.
Materials:
- Early-stage human embryos (appropriate ethical approvals required)
- "Light-caged" molecular constructs (e.g., photolyzable linkers for cell adhesion proteins)
- Confocal or multi-photon microscopy system with precise light control
- Micro-pipettes for embryo immobilization
- Environmental chamber maintaining 37°C and 5% CO₂
- Immunofluorescence staining reagents for developmental markers
Procedure:
- Embryo Preparation: Culture embryos to the desired pre-gastrulation stage under standard conditions.
- Molecular Loading: Introduce light-sensitive "caged" molecules targeting cell adhesion complexes using microinjection or viral transduction.
- Force Application: Apply focused light patterns (wavelength 350-450 nm) to specific embryonic regions using a digital micromirror device to uncage molecules and locally alter intercellular mechanics.
- Real-time Imaging: Capture embryo morphology every 20 seconds using multiview selective plane illumination microscopy (MuVi-SPIM) to track deformation dynamics [77].
- Force Quantification: Use traction force microscopy or laser ablation to measure resulting tissue tensions and cell-shape changes.
- Outcome Assessment: Fix embryos at specific timepoints and perform immunofluorescence for germ layer markers (e.g., Brachyury for mesoderm) to correlate mechanical inputs with cell fate decisions.
- Data Analysis: Quantify axis elongation, curvature changes, and gene expression patterns relative to light stimulation parameters.
Protocol 2: Analyzing Molecular Triggers of Gastrulation
Purpose: To investigate how extracellular presentation of Syntaxin4 regulates region-specific gastrulation initiation.
Materials:
- Mouse embryonic egg cylinders (E6.0) or embryonic stem cells (ESCs)
- Membrane-impermeable antagonistic peptides against extracellular Stx4
- Small-molecule inhibitors/activators (targeting FAK, AKT/PI3K, Rho/ROCK pathways)
- Immunocytochemistry reagents for P-cadherin and Brachyury
- Live-cell imaging setup for morphological tracking
- Western blot equipment for phosphorylation analysis
Procedure:
- Model Preparation: Isolate mouse embryonic egg cylinders or differentiate ESCs toward epiblast-like states.
- Pathway Perturbation: Treat samples with Stx4 antagonistic peptides or pathway-specific small molecules (e.g., FAK inhibitor at 10µM).
- Signaling Analysis: Harvest cells at timed intervals for Western blotting to assess FAK dephosphorylation, AKT/PI3K activity, and Rho/ROCK activation.
- Morphological Assessment: Fix samples and stain for P-cadherin redistribution and actin cytoskeleton organization.
- Lineage Tracking: Quantify Brachyury-positive cells via immunostaining or GFP reporters as a marker of mesendodermal commitment.
- Functional Validation: Perform rescue experiments by constitutive activation of downstream effectors (e.g., constitutively active Rho) in the presence of Stx4 inhibition.
Signaling Pathways and Experimental Workflows
Stx4/FAK Signaling Pathway in Gastrulation Initiation
Figure 1: Molecular pathway of Stx4-mediated gastrulation initiation
Optogenetic Workflow for Gastrulation Research
Figure 2: Experimental workflow for optogenetic gastrulation studies
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for Gastrulation Mechanics Studies
| Reagent / Tool | Function & Mechanism | Example Applications |
|---|---|---|
| Optogenetic "Caged" Molecules [76] | Light-sensitive compounds that alter cell adhesion properties upon illumination | Precise spatiotemporal control of mechanical forces in developing embryos |
| Membrane-Impermeable Stx4 Peptides [78] | Antagonists that block extracellular Syntaxin4 function | Investigating initiation of region-specific gastrulation events |
| Small-Molecule FAK Inhibitors [78] | Pharmacological agents that deactivate Focal Adhesion Kinase signaling | Dissecting mechanical transduction pathways in gastrulation |
| MuVi-SPIM Microscopy [77] | Multiview Selective Plane Illumination Microscopy for high-resolution 3D imaging | Long-term live imaging of entire embryos during morphogenesis |
| Microfluidic Cell Culture Platforms [79] [7] | Miniaturized devices for precise cellular microenvironment control | Modeling early developmental niches and tissue interactions |
| Rho/ROCK Pathway Modulators [78] | Chemical activators/inhibitors of cytoskeletal contractility | Probing force generation mechanisms in cell shape changes |
The integration of optogenetic force control with molecular gastrulation studies creates a powerful platform for translating developmental mechanics into clinical insights. Understanding how mechanical forces guide cell internalization during gastrulation provides a framework for addressing developmental disorders and advancing regenerative strategies. The tools and protocols detailed here enable researchers to not only decipher fundamental principles of embryogenesis but also envision novel therapeutic approaches that harness mechanical cues for tissue engineering and disease modeling. As these technologies mature, they hold promise for informing strategies in organoid development, regenerative medicine, and the treatment of congenital conditions rooted in early embryonic patterning errors.
Conclusion
The integration of optogenetics into the study of gastrulation marks a significant leap forward, moving research from passive observation to active, precise manipulation of developmental processes. The key takeaways are the critical interdependence of biochemical signals and mechanical forces in axis formation, the unparalleled spatiotemporal control offered by light-sensitive tools, and the ability to define critical developmental windows with minimal off-target effects. Future efforts must focus on refining these tools for lower toxicity and deeper tissue penetration, and on leveraging them to build more accurate models of human development. The path forward is bright, with the potential to illuminate not only the fundamentals of life's beginnings but also to inform novel regenerative and fertility therapies.
References
- 1. Improved model system allows researchers to study ... [https://www.sciencedaily.com/releases/2025/05/250515131716.htm]
- 2. Mechanical forces are key to embryonic self-organization [https://www.rockefeller.edu/news/38639-mechanical-forces-are-key-to-embryonic-self-organization]
- 3. Study uncovers role of mechanical forces in human ... [https://www.news-medical.net/news/20251122/Study-uncovers-role-of-mechanical-forces-in-human-gastrulation.aspx]
- 4. Migrating mesoderm cells self-organize into a dynamic ... [https://pubmed.ncbi.nlm.nih.gov/40839526/]
- 5. Migrating mesoderm cells self-organize into a dynamic ... [https://elifesciences.org/articles/84749]
- 6. Optogenetic control of Nodal signaling patterns - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12070070/]
- 7. Advances in engineered models of peri-gastrulation [https://www.sciencedirect.com/science/article/pii/S2589004225009204]
- 8. Highly dynamic mechanical transitions in embryonic cell ... [https://www.nature.com/articles/s41467-025-61702-4]
- 9. of multiple Internalization C. elegans cells ... during gastrulation [https://pmc.ncbi.nlm.nih.gov/articles/PMC3022094/]
- 10. Actomyosin-based Self-organization of cell ... internalization during [https://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-10-94]
- 11. Quantitative imaging of collective cell migration during ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2854020/]
- 12. Optogenetic Methods to Control Tissue Mechanics in Drosophila | SpringerLink [https://link.springer.com/protocol/10.1007/978-1-0716-2541-5_13]
- 13. Optogenetics and Light-Sheet Microscopy - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK599195/]
- 14. A minimal-complexity light-sheet microscope maps network ... [https://www.nature.com/articles/s41598-022-24350-y]
- 15. Advances in mechanochemical modelling of vertebrate ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12409983/]
- 16. Crosstalk between tissue mechanics and BMP4 signaling ... [https://www.sciencedirect.com/science/article/abs/pii/S1934590925003376]
- 17. Endogenous OptoRhoGEFs reveal biophysical principles ... [https://www.nature.com/articles/s41467-025-62483-6]
- 18. Characterization of Optimal Optogenetic Stimulation ... [https://www.eneuro.org/content/12/9/ENEURO.0220-25.2025]
- 19. 综述:光é—ä¼ å¦ä½œä¸ºæŽ§åˆ¶é¸¡èƒšèƒŽå‘生过程ä¸å¯å…´å¥‹ä¸Žä¸å¯ ... [https://www.ebiotrade.com/newsf/2025-10/20251010172318017.htm]
- 20. æœä¹…林组æˆåŠŸå¼€å‘光控å•ç¥žç»å…ƒå¤šæ¨¡æ€æ ‡è®°æŠ€æœ¯ [https://www.ion.ac.cn/xwen/kyjz/2025/202508/t20250822_7909184.html]
- 21. 生物å‘光光é—ä¼ å¦2.0:利用生物å‘光在体外和体内激活光æ•è›‹ç™½ [https://www.jove.com/cn/t/62850/bioluminescent-optogenetics-20-harnessing-bioluminescence-to-activate]
- 22. Optical control of biological processes by light-switchable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4529752/]
- 23. Optogenetic activation of intracellular signaling based on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8451544/]
- 24. Understanding CRY2 interactions for optical control of ... [https://www.nature.com/articles/s41467-017-00648-8]
- 25. The Dual Characteristics of Light-Induced Cryptochrome 2, ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5061508/]
- 26. Spatiotemporal Control of Cell Signalling Using A Light ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2989900/]
- 27. Deconstructing and repurposing the light-regulated ... [https://www.nature.com/articles/s42003-019-0687-9]
- 28. Optogenetic control of protein binding using light ... [https://www.nature.com/articles/s41467-020-17836-8]
- 29. Researchers Use Light-Controlled Embryos to Study ... [https://www.geneonline.com/researchers-use-light-controlled-embryos-to-study-mechanical-forces-during-gastrulation-in-early-human-development/]
- 30. Protein design accelerates the development and ... [https://www.sciencedirect.com/science/article/pii/S2001037025000431]
- 31. Potent optogenetic regulation of gene expression in ... [https://pubmed.ncbi.nlm.nih.gov/40586302/]
- 32. Designed optogenetic tool for bridging single-neuronal ... [https://www.nature.com/articles/s41467-025-62938-w]
- 33. Light-Activated DNA G-Quadruplex Controls Gene ... [https://bioengineer.org/light-activated-dna-g-quadruplex-controls-gene-expression/]
- 34. Development of label-free light-controlled gene expression ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2024.1324757/full]
- 35. Optogenetic protein clustering through fluorescent ... [https://www.nature.com/articles/s41467-017-00060-2]
- 36. Light-Induced Protein Clustering for Optogenetic ... [https://www.mdpi.com/2218-273X/9/2/61]
- 37. The surprising way metabolism controls embryo growth [https://www.sciencedaily.com/releases/2025/09/250920214451.htm]
- 38. In a new study, scientists in the Brivanlou lab have used ... [https://www.facebook.com/rockefelleruniversity/posts/in-a-new-study-scientists-in-the-brivanlou-lab-have-used-light-inducible-gene-ex/1258118046358480/]
- 39. Activation of Bone Morphogenetic Protein 4 Signaling Leads ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3103383/]
- 40. Temporal BMP4 effects on mouse embryonic and ... [https://www.nature.com/articles/s41586-024-07937-5]
- 41. Combinatorial BMP4 and activin direct the choice between ... [https://www.sciencedirect.com/science/article/pii/S1534580725005295]
- 42. Mechanical forces direct stem cell behaviour in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5803560/]
- 43. Spatiotemporal, optogenetic control of gene expression in ... [https://www.nature.com/articles/s41592-023-01986-w]
- 44. Optogenetic control of Wnt signaling models cell-intrinsic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10399980/]
- 45. Gastruloids: Pluripotent stem cell models of mammalian ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9477185/]
- 46. The emergence of human gastrulation upon in vitro attachment [https://pmc.ncbi.nlm.nih.gov/articles/PMC10828709/]
- 47. Flipping the switch on disease: Texas A&M scientists pioneer ... [https://stories.tamu.edu/news/2025/09/09/flipping-the-switch-on-disease-texas-am-scientists-pioneer-light-controlled-genetic-tools/]
- 48. New light-based technology arranges cellular messengers ... [https://www.news-medical.net/news/20251118/New-light-based-technology-arranges-cellular-messengers-with-unprecedented-precision.aspx]
- 49. Live imaging and quantitative analysis of gastrulation in ... [https://pubmed.ncbi.nlm.nih.gov/24525751/]
- 50. A quantitative method for measuring phototoxicity of a live ... [https://pubmed.ncbi.nlm.nih.gov/22341230/]
- 51. Exploring Mitochondrial Phototoxicity: Mechanisms, ... [https://www.sciencedirect.com/science/article/abs/pii/S030090842500149X]
- 52. Optimizing the in vitro neuronal microenvironment to mitigate ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04591-0]
- 53. Optimizing the in vitro neuronal microenvironment to ... [https://pubmed.ncbi.nlm.nih.gov/40999471/]
- 54. An improved method for differentiating cell-bound from ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4522237/]
- 55. Imaging flow cytometry reveals the mechanism of equine ... [https://www.nature.com/articles/s41598-025-87080-x]
- 56. Minimal videos: Trade-off between spatial and temporal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7330814/]
- 57. A study on trade-offs between spatial resolution and ... [https://www.sciencedirect.com/science/article/pii/S0303243419306439]
- 58. How do tradeoffs in satellite spatial and temporal resolution ... [https://tc.copernicus.org/articles/17/2629/2023/]
- 59. Divergent evolutionary strategies pre-empt tissue collision ... [https://www.nature.com/articles/s41586-025-09447-4]
- 60. Light sheet fluorescence microscopy for monitoring drug ... [https://www.sciencedirect.com/science/article/pii/S0169409X25000055]
- 61. Light and electron microscopy continuum-resolution imaging ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10114294/]
- 62. A 3D spheroid model for assessing nanocarrier-based ... [https://www.nature.com/articles/s44385-025-00041-x]
- 63. An optimized optogenetic clustering tool for probing protein ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4170572/]
- 64. Kinetics of the LOV domain of ZEITLUPE determine its ... [https://elifesciences.org/articles/21646]
- 65. Kinetics of Conformational Changes of the FKF1-LOV ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2998605/]
- 66. In-cell infrared difference spectroscopy of LOV photoreceptors ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7450117/]
- 67. 通过光é—ä¼ å˜æž„调控对蛋白质活性进行时空调控 [https://www.jove.com/cn/t/67261/spatiotemporal-control-protein-activity-through-optogenetic]
- 68. Spatiotemporal control of cell–cell reversible interactions ... [https://www.nature.com/articles/ncomms13088]
- 69. Recent Advances in Spatiotemporal Manipulation of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12067681/]
- 70. A spatiotemporal atlas of mouse gastrulation and early ... [https://pubmed.ncbi.nlm.nih.gov/40728928/]
- 71. 汪贻广团队å‘展pH超æ•æ„ŸåŒºé—´æˆåƒæ–°ç–ç•¥ - 北京大å¦è¯å¦é™¢ [https://sps.bjmu.edu.cn/xyxw/a78889b8d33f4efdb5ef0bd54e81f203.htm]
- 72. Control of spatio-temporal patterning via cell growth in a ... [https://www.nature.com/articles/s41467-024-53078-8]
- 73. Recent advances in spatiotemporal control of the CRISPR/ ... [https://www.sciencedirect.com/science/article/abs/pii/S0927776524007331]
- 74. Advances in engineered models of peri-gastrulation - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12159512/]
- 75. Embryo model completes gastrulation to neurulation and ... [https://www.nature.com/articles/s41586-022-05246-3]
- 76. 光控技術æ示機械力在早期人類胚胎發育ä¸çš„é—œéµä½œç”¨ [https://geneonline.news/%E5%85%89%E6%8E%A7%E6%8A%80%E8%A1%93%E6%8F%AD%E7%A4%BA%E6%A9%9F%E6%A2%B0%E5%8A%9B%E5%9C%A8%E6%97%A9%E6%9C%9F%E4%BA%BA%E9%A1%9E%E8%83%9A%E8%83%8E%E7%99%BC%E8%82%B2%E4%B8%AD%E7%9A%84%E9%97%9C%E9%8D%B5/]
- 77. Embryo-scale tissue mechanics during Drosophila ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4846315/]
- 78. Extracellular presentation of syntaxin4 as a potential trigger ... [https://pubmed.ncbi.nlm.nih.gov/41161740/]
- 79. Progress and prospect of separation and analysis of single ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12059993/]