Optimized IVF Protocol for Timed Embryo Donor Mice: Enhancing Precision in Reproductive Research
This article provides a comprehensive guide to in vitro fertilization (IVF) protocols specifically tailored for generating timed embryo donor mice, a critical resource for biomedical and drug development research.
Optimized IVF Protocol for Timed Embryo Donor Mice: Enhancing Precision in Reproductive Research
Abstract
This article provides a comprehensive guide to in vitro fertilization (IVF) protocols specifically tailored for generating timed embryo donor mice, a critical resource for biomedical and drug development research. It covers the foundational principles of mouse reproductive biology and the importance of precise timing in superovulation. The content details a step-by-step methodological application, including reagent preparation, oocyte collection, and sperm handling. It further addresses common troubleshooting scenarios and optimization strategies, such as strain-specific adjustments and novel sperm preparation techniques. Finally, it explores validation methods and compares efficiency across different mouse strains, offering researchers a robust framework to improve experimental reproducibility and success rates in generating embryo donors.
Fundamentals of Mouse Reproductive Biology and Timed Donor Principles
The Role of Mouse Models in Advancing Reproductive Biology and Drug Development
Mouse models serve as an indispensable cornerstone of biomedical research, providing critical insights into the complex mechanisms governing mammalian reproduction and enabling the development of novel therapeutic strategies. Their genetic similarity to humans, short reproductive cycles, and the availability of sophisticated genetic engineering tools make them particularly valuable for studying reproductive biology and advancing drug development. This article presents a detailed overview of current applications and methodologies, with a specific focus on in vitro fertilization (IVF) protocols for timed embryo donor mice, framed within the context of a broader thesis on reproductive technologies. We provide structured experimental data, detailed protocols, and visual workflows specifically designed for researchers, scientists, and drug development professionals working at the intersection of reproductive biology and translational medicine.
Key Advances in Reproductive Biology Using Mouse Models
Foundational Research and Therapeutic Development
Recent research utilizing mouse models has yielded significant advances across multiple domains of reproductive biology, from addressing infertility to developing novel contraceptive strategies and refining assisted reproductive technologies (ART). The following table summarizes key quantitative findings from recent studies.
Table 1: Key Quantitative Findings from Recent Mouse Model Studies in Reproductive Biology
| Research Area | Key Finding | Quantitative Result | Significance | Citation |
|---|---|---|---|---|
| Infertility Treatment | mRNA therapy restores spermatogenesis in NOA mice | 22.2% live birth rate (26 pups/117 embryos) via ICSI | Offers non-integrating gene therapy approach for genetic infertility | [1] |
| Fertility Enhancement | Fertilin peptide improves embryo development and birth rates | Accelerated blastocyst expansion by 2h43; Significant improvement in live birth rate | Potential therapeutic for improving IVF outcomes | [2] |
| Contraceptive Research | Human PRSS55 rescues infertility in knockout mice | RES TM line showed fertility comparable to wild-type controls | Validates model for testing human PRSS55-targeted contraceptives | [3] |
| ART Safety | Mutation rates in IVF-conceived mice | ~30% more single-nucleotide variants vs. natural conception | Informs safety assessment of assisted reproductive technologies | [4] |
| Germ-Free Production | Optimized cesarean technique improves fetal survival | FRT-CS significantly improved survival while maintaining sterility | Enhances efficiency of germ-free mouse production for microbiome studies | [5] |
Humanized Models for Drug Development
The development of humanized mouse models has proven particularly transformative for preclinical drug development. For instance, Cyagen's C3 and C5 humanized mouse models, created via precise gene replacement technology, express the full human C3 or C5 genes while suppressing the endogenous mouse genes. These models have become foundational for testing highly specific biologics, such as siRNA-based therapeutics and bispecific antibodies, whose efficacy is dependent on human protein targets [6]. This approach directly addresses the translational bottleneck caused by species-specific interactions between therapeutic candidates and their targets, thereby providing more reliable predictive data for human clinical outcomes.
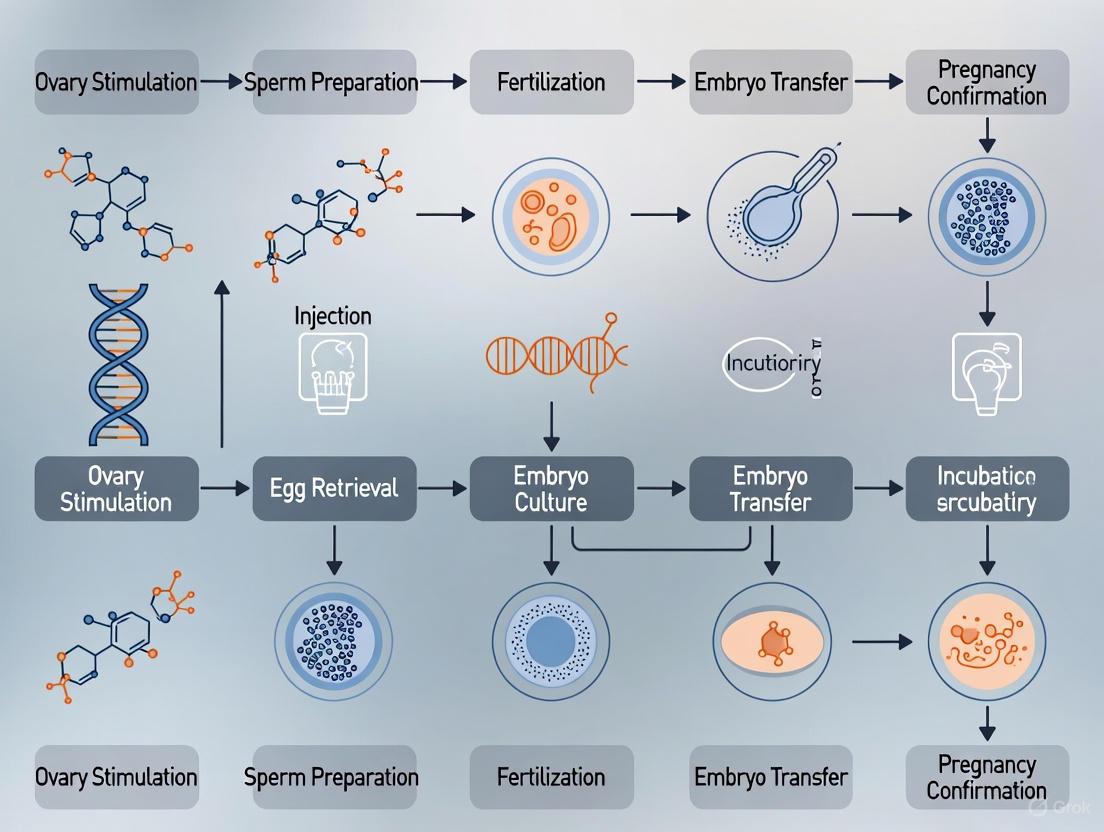
Detailed In Vitro Fertilization Protocol for Timed Embryo Donor Mice
This section provides a comprehensive, step-by-step protocol for obtaining timed embryos from donor mice via IVF, incorporating best practices and optimizations from core facility procedures and recent research.
Pre-IVF Procedures and Animal Preparation
Donor Mouse Selection and Superovulation
- Donor Females: Utilize 30-50 female mice, 3-5 weeks old, from the line of interest. Younger females typically yield higher oocyte quantities and quality [7].
- Superovulation Hormone Regimen:
- Day 1 (Afternoon): Administer intraperitoneal (IP) injection of pregnant mare serum gonadotropin (PMS/PMSG) at 5-7.5 IU per mouse to stimulate follicular growth.
- Day 3 (Afternoon): Administer IP injection of human chorionic gonadotropin (hCG) at 5-7.5 IU per mouse to induce final oocyte maturation [2] [7].
- Sperm Donors: Provide 1-2 proven breeder male mice (2-5 months old) for fresh sperm collection. Homozygous males are preferred if maintaining a specific genetic background [7].
- Euthanize one male sperm donor by cervical dislocation or CO₂ asphyxiation following approved IACUC protocols.
- Quickly dissect to isolate the cauda epididymides.
- Transfer epididymides to a 1.5 mL microcentrifuge tube containing 500 µL of pre-warmed, equilibrated human tubal fluid (HTF) medium supplemented with 4 mg/mL bovine serum albumin (BSA).
- Make several incisions in the epididymal tubules using fine scissors or a 25-gauge needle to allow sperm to swim out.
- Incubate the sperm suspension for 45-90 minutes at 37°C under 5% CO₂ to allow for capacitation. Assess sperm motility and concentration after incubation [7].
Oocyte Collection and Fertilization
Oocyte Harvesting
- Approximately 13-15 hours post-hCG injection, euthanize the superovulated donor females.
- Collect the oviducts and place them in a dish of pre-warmed HEPES-buffered medium (e.g., M2 or FHM).
- Under a stereomicroscope, locate the ampulla, a swollen section of the oviduct containing the cumulus-oocyte complexes (COCs).
- Puncture the ampulla with a fine needle to release the COCs into the medium.
In Vitro Fertilization
- Transfer 100-200 µL of the capacitated sperm suspension (containing ~1-5 x 10⁶ sperm/mL) into a 100-200 µL drop of fertilization medium (e.g., HTF with BSA) under mineral oil.
- Wash the collected COCs briefly and transfer 20-30 oocytes into the fertilization drop containing sperm.
- Co-incubate gametes for 4-6 hours at 37°C under 5% CO₂ [7].
Post-Fertilization Procedures and Embryo Culture
Embryo Washing and Culture
- After co-incubation, wash the presumptive zygotes thoroughly through several drops of pre-equilibrated KSOM or other embryo culture medium to remove adherent sperm and cumulus cells.
- Transfer 20-25 zygotes into a 50-100 µL drop of fresh KSOM medium under mineral oil.
- Culture embryos at 37°C under 5% CO₂, assessing development daily. Under optimal conditions, embryos should progress to the 2-cell stage by 24 hours post-fertilization and to the blastocyst stage by days 3.5-4.5 [7].
Embryo Transfer or Cryopreservation
- For embryo transfer, surgically transfer 10-15 two-cell embryos (at E0.5) into the oviducts of a pseudo-pregnant 0.5 days post-coitum (dpc) recipient female, or transfer blastocysts (at E3.5) into the uterus of a 2.5 dpc recipient [7].
- For cryopreservation, cryopreserve 2-cell, morula, or blastocyst-stage embryos using controlled-rate freezing or vitrification protocols for long-term storage [7].
Table 2: IVF Project Timeline and Key Milestones (Adapted from MART Core Protocol)
| Timeline | Activity | Key Steps and Notes |
|---|---|---|
| Time depends on colony size | Breeding and Mouse Preparation | Breed to obtain 5-10 male and 20-30 female donors (5-6 weeks old). |
| Day 1-3 | Hormone Priming of Donors | Inject PMS (Day 1) and hCG (Day 3). |
| Day 4 | Check Mating Plugs & Sperm Prep | Confirm superovulation; Collect and capacitate sperm. |
| Day 5 | Oocyte Collection & IVF | Harvest oocytes; Perform in vitro fertilization (4-6 hr co-incubation). |
| Day 6 | Embryo Check and Culture | Confirm 2-cell embryos; Continue culture to morula/blastocyst if needed. |
| Day 7 Onwards | Embryo Transfer or Cryopreservation | Transfer to pseudo-pregnant recipients or cryopreserve for future use. |
Experimental Data and Workflow Visualization
IVF and Rederivation Workflow
The following diagram illustrates the complete workflow for generating pathogen-free mice via IVF rederivation, a critical process for importing or safeguarding valuable genetic lines.
Diagram 1: IVF Rederivation Workflow for Pathogen-Free Mice
mRNA Therapy for Male Infertility
The diagram below outlines the innovative mechanism of using lipid nanoparticles (LNPs) to deliver mRNA for treating non-obstructive azoospermia (NOA) in a mouse model, a groundbreaking therapeutic approach.
Diagram 2: mRNA Therapy Mechanism for Male Infertility
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents for Mouse Reproductive Biology and IVF
| Reagent/Material | Function/Application | Example/Notes |
|---|---|---|
| Pregnant Mare Serum Gonadotropin (PMS/PMSG) | Hormonal priming for superovulation in donor females. | Analogous to FSH; stimulates follicular development. [2] [7] |
| Human Chorionic Gonadotropin (hCG) | Triggers final oocyte maturation and ovulation. | Analogous to LH; administered after PMS. [2] [7] |
| HTF (Human Tubal Fluid) Medium | Standard medium for in vitro fertilization and sperm capacitation. | Often supplemented with BSA (4 mg/mL). [7] |
| KSOM Medium | Serum-free, optimized medium for post-fertilization embryo culture. | Supports development from zygote to blastocyst. [7] |
| M2/FHM Medium | HEPES-buffered holding media for oocyte collection and manipulations outside a CO₂ incubator. | Maintains physiological pH outside the incubator. [7] |
| Lipid Nanoparticles (LNPs) | Delivery vehicle for mRNA-based therapies to restore gene function. | Used to deliver Pdha2 mRNA to testis in NOA model. [1] |
| Clidox-S | Chlorine dioxide disinfectant for sterilizing tissues and equipment in germ-free isolators. | Used at 1:3:1 dilution, activated for 15 min before use. [5] |
| Fertilin Peptide | Synthetic peptide that mimics sperm-binding molecule; accelerates embryo development. | Used at 100 μM concentration to improve blastocyst formation rates. [2] |
Mouse models continue to be pivotal in unraveling the complexities of mammalian reproductive biology and accelerating the development of novel therapeutics. The refined IVF protocols, quantitative data, and visual workflows presented in this article provide a robust resource for researchers engaged in both fundamental reproductive science and translational drug development. As the field progresses, the integration of advanced technologies—such as humanized models for target validation, mRNA-based therapies for genetic infertility, and optimized protocols for generating specialized animal models—will undoubtedly deepen our understanding of reproductive mechanisms and enhance our ability to address reproductive challenges in human health.
The estrous cycle represents a series of physiologically recurring changes induced by reproductive hormones in most female mammals, crucial for timed reproduction and fertilization success [8]. In laboratory mice, a profound understanding of this cycle is indispensable for reproductive research, particularly in the generation of timed embryo donors for in vitro fertilization (IVF) studies. The mouse is polyestrous, experiencing cycles every 4-5 days throughout the year without seasonal influence, making it a consistent model for research [9]. The cycle is divided into four distinct stages—proestrus, estrus, metestrus, and diestrus—each characterized by specific hormonal, ovarian, and vaginal cytological changes [9] [8]. Mastery of these stages allows researchers to precisely time experimental interventions, ensuring optimal oocyte yield and quality for procedures such as superovulation and IVF, which are foundational for preserving genetically engineered models and biomedical discovery [5] [10].
Table 1: Stages of the Murine Estrous Cycle
| Stage | Duration (Hours) | Key Hormonal Features | Vaginal Cytology | Behavioral/Sexual Status |
|---|---|---|---|---|
| Proestrus | ~12 | Follicles grow, estrogen rises | Nucleated epithelial cells | Not sexually receptive |
| Estrus | ~12 | Estrogen peak, pre-ovulatory LH surge | Cornified (squamous) epithelial cells | Sexually receptive ("in heat") |
| Metestrus | ~21 | Corpus luteum begins to form | Leukocytes appear with nucleated and cornified cells | Not receptive |
| Diestrus | ~57 | Progesterone from corpus luteum dominates | Predominantly leukocytes | Not receptive |
Hormonal Control of the Estrous Cycle
The estrous cycle is governed by an exquisitely sensitive feedback system, the Hypothalamic-Pituitary-Ovarian (HPO) Axis, which coordinates the timing of ovarian events with the preparation of the reproductive tract to maximize the chances of successful fertilization and pregnancy [9] [11].
The Hypothalamic-Pituitary-Ovarian (HPO) Axis
The hypothalamus secretes Gonadotropin-Releasing Hormone (GnRH) in a pulsatile manner, which in turn stimulates the anterior pituitary gland to secrete the gonadotropins Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) [11]. These gonadotropins act directly on the ovaries. FSH is primarily responsible for the recruitment and development of a cohort of ovarian follicles, while LH stimulates theca cells to produce androgens, which are subsequently aromatized into estrogens by granulosa cells—a process known as the two-cell, two-gonadotropin hypothesis of estrogen synthesis [12]. The ovarian hormones, estradiol and progesterone, then complete the feedback loop by exerting both negative and positive feedback on the hypothalamus and pituitary to regulate GnRH, FSH, and LH secretion [12] [9].
Figure 1: The Hypothalamic-Pituitary-Ovarian (HPO) Axis. This diagram illustrates the core hormonal feedback loops that regulate the estrous cycle. GnRH from the hypothalamus stimulates the pituitary to release FSH and LH, which act on the ovary. Ovarian hormones (estradiol, progesterone) complete the loop via feedback. CL = Corpus Luteum.
Hormonal Dynamics During the Follicular and Luteal Phases
The cycle can be broadly divided into two phases: the follicular phase (encompassing proestrus and estrus) and the luteal phase (metestrus and diestrus) [12] [9].
During the follicular phase, the decline of progesterone from the previous cycle's corpus luteum allows FSH to rise, recruiting a group of follicles [12]. One follicle becomes "dominant" and secretes increasing amounts of estradiol. This rising estradiol exerts negative feedback on FSH (preventing other follicles from maturing) and, upon reaching a sustained threshold (>200 pg/mL for ~50 hours), triggers a positive feedback surge of LH [12]. This LH surge is the definitive signal that induces ovulation, which occurs approximately 10-12 hours later in mice [9].
Following ovulation, the luteal phase begins. The ruptured follicle transforms into the corpus luteum, a temporary endocrine structure that secretes large quantities of progesterone [12] [11]. Progesterone's primary role is to prepare the uterine lining for implantation. If pregnancy does not occur, the corpus luteum undergoes luteolysis, progesterone levels fall, and the cycle begins anew with a rise in FSH [12] [8].
Table 2: Key Hormone Production Rates During the Mouse Estrous Cycle
| Sex Steroid | Early Follicular (µg/24h) | Preovulatory (µg/24h) | Mid-Luteal (µg/24h) |
|---|---|---|---|
| Estradiol | 36 | 380 | 250 |
| Progesterone (mg/24h) | 1 | 4 | 25 |
| Testosterone | 144 | 171 | 126 |
The Principle of Superovulation
Superovulation is a controlled hormonal technique used to override the natural limits of the estrous cycle, inducing the maturation and ovulation of a significantly larger number of oocytes than occurs spontaneously [13] [14]. This is critical for maximizing embryo yield in IVF and other assisted reproductive technologies, especially when working with valuable or genetically modified donor strains [5].
The physiological basis for superovulation lies in mimicking and amplifying the natural hormonal sequence of the follicular phase. In a natural cycle, rising FSH recruits a cohort of follicles, but only one is selected to become dominant and ovulate, while the others undergo atresia [12]. Superovulation involves the exogenous administration of equine-derived Gonadotropins, which have high FSH-like activity, to stimulate the synchronous development of a larger cohort of follicles [14]. This is followed by an injection of human Chorionic Gonadotropin (hCG), which acts as a potent analog of LH, to trigger the final maturation and ovulation of all the recruited follicles simultaneously [13] [14]. The timing of this protocol is precisely coordinated with the donor's estrous cycle to achieve the highest quality and quantity of oocytes.
Detailed Superovulation and IVF Protocol for Timed Embryo Donor Mice
This protocol integrates established methodologies from leading reproductive laboratories to ensure high efficiency and reproducibility [13] [14] [10]. Adherence to precise timing is paramount.
Reagent and Material Preparation
Table 3: The Scientist's Toolkit - Essential Reagents for Superovulation and IVF
| Category/Item | Specific Examples | Function & Rationale |
|---|---|---|
| Hormones for Superovulation | Pregnant Mare Serum Gonadotropin (PMSG), Human Chorionic Gonadotropin (hCG) | PMSG (eCG) mimics FSH for multi-follicular growth; hCG mimics the LH surge to trigger ovulation. |
| Fertilization Media | Human Tubal Fluid (HTF), CARD MEDIUM, Modified RVF (mRVF) | Provides energy sources and ionic composition mimicking the oviductal environment to support sperm capacitation, fertilization, and early zygote formation. |
| Sperm Handling Media | FERTIUP | A pre-incubation medium designed to support sperm capacitation and maintain motility before introduction to oocytes. |
| Embryo Culture Media | KSOM-AA, M16 | Complex media supporting preimplantation embryo development from zygote to blastocyst stage. |
| General Supplies | Embryo-tested mineral oil, 35/60mm culture dishes, pulled glass pipettes, CO2 incubator | Oil overlays prevent evaporation; specific dishes and tools allow for precise handling of gametes and embryos. |
Step-by-Step Protocol
Figure 2: Superovulation and IVF Workflow. A visual guide to the critical path and timing for generating timed embryo donors.
Day 1: Initiation of Follicular Growth
- Select 3- to 4-week-old female mice (immature donors have a higher superovulation response) [10].
- Administer an intraperitoneal (IP) injection of 5 IU of PMSG between 2:00 PM and 6:00 PM [13]. This timing helps align ovulation with the natural circadian rhythm.
Day 3: Triggering Ovulation
- Precisely 48 hours after the PMSG injection, administer an IP injection of 5 IU of hCG [13] [14].
- Prepare all necessary fertilization media dishes (e.g., sperm dish with FERTIUP, fertilization dish with CARD MEDIUM or HTF+, washing dishes with mHTF) and equilibrate them in a CO2 incubator at least 30 minutes before use [13].
Day 4: In Vitro Fertilization (13-17 hours post-hCG)
- Oocyte Collection: Sacrifice donor females 13-17 hours after hCG injection [14] [15]. Rapidly dissect the oviducts and place them in the fertilization dish under oil. Using fine forceps, tear the swollen ampulla to release the cumulus-oocyte complexes (COCs). Transfer the COCs to the equilibrated fertilization drop. Critical Note: The interval from euthanasia to oocyte collection must be minimized (optimally <3 minutes) to prevent zona pellucida hardening and maintain high fertilization rates [15].
- Sperm Preparation:
- For Fresh Sperm: Sacrifice a proven male, remove the cauda epididymides and vasa deferentia, and release sperm into a pre-equilibrated drop of FERTIUP. Incubate for 30-60 minutes for capacitation [13] [14].
- For Frozen/Thawed Sperm: Rapidly thaw a frozen straw or vial in a 37°C water bath. For straws, expel contents directly into a FERTIUP drop. For vials, the sperm suspension can be washed or transferred directly to FERTIUP. Incubate for 30 minutes [13] [14].
- Insemination: Using a wide-bore pipette tip to avoid damaging sperm, add a 3-10 µL aliquot of the motile sperm suspension to the fertilization drop containing the COCs [13] [14]. The final motile sperm concentration should be approximately 1-2.5 x 10^6/mL [14].
- Co-incubation and Washing: Place the fertilization dish back in the incubator (37°C, 5% CO2) for 4-6 hours. Subsequently, wash the oocytes through several drops of fresh medium (e.g., mHTF or KSOM-AA) to remove adherent sperm and cellular debris [14].
Day 5: Assessment and Embryo Culture
- The following morning (approximately 24 hours post-insemination), examine the oocytes under a microscope. Successfully fertilized oocytes will have cleaved to the 2-cell stage [14].
- Transfer the 2-cell embryos to a fresh culture dish with KSOM-AA medium for further development to the blastocyst stage, or surgically transfer them into 0.5 days post coitum (dpc) pseudopregnant recipient females to generate live offspring [13] [14] [5].
Critical Factors for Protocol Success
- Strain Background: The C57BL/6 strain, the most common genetic background for engineered models, is known for lower and more variable superovulation and IVF efficiency compared to outbred or hybrid strains [10]. The use of optimized media like CARD MEDIUM or HTF+ (HTF supplemented with extra Calcium and Glutathione) can significantly improve fertilization rates in these difficult strains [13] [10].
- Sperm Source and Quality: Frozen-thawed sperm often exhibits reduced motility and fertility compared to fresh sperm. Protocols for frozen sperm may require modifications, such as omitting the pre-incubation capacitation step or using cumulus-denuded oocytes to facilitate fertilization [14].
- Predictability and Efficiency: Integrating IVF into the production pipeline for germ-free mice via cesarean section allows for precise control over the donor's delivery date, greatly enhancing experimental reproducibility and efficiency compared to reliance on natural mating [5].
The generation of a "Timed Donor" in mouse embryology research refers to the precise synchronization of donor embryo developmental stage with the experimental requirements of the host system. This precision is paramount because embryonic development proceeds along a highly orchestrated timeline, and even minor discrepancies in developmental age can compromise experimental outcomes. The core principle hinges on isochronic transplantation, where the developmental stage of donor cells or embryos must be meticulously matched with that of the host to ensure functional integration and accurate research data [16]. The failure to achieve this synchrony, known as heterochronic injection, reliably results in the failure of chimera formation, underscoring that matching developmental age is a non-negotiable prerequisite for successful engraftment [16]. This document outlines the standardized protocols and application notes for establishing precisely timed embryo donor mice, a critical foundation for research in developmental biology, genetic engineering, and regenerative medicine.
Mouse Embryo Developmental Staging: A Reference Timeline
A precise understanding of the murine embryo developmental sequence is the first step in defining the timed donor. The following table summarizes the key morphological stages and their corresponding temporal landmarks post-fertilization.
Table 1: Standard Timeline of Preimplantation Mouse Embryo Development In Vivo
| Day Post-Coitum | Developmental Stage | Key Morphological Characteristics |
|---|---|---|
| 0.5 (E0.5) | Fertilization (Zygote) | Formation of male and female pronuclei [17] [18]. |
| 1.5 (E1.5) | 2-Cell Embryo | First cleavage division [17]. |
| 2.0 (E2.0) | 4-Cell Embryo | Second cleavage division [17]. |
| 2.5 (E2.5) | 8-Cell Embryo | Third cleavage division; onset of compaction [17]. |
| 3.5 (E3.5) | Morula | A compact ball of 16+ cells; cell boundaries become indistinct [18]. |
| 4.5 (E4.5) | Blastocyst | Formation of fluid-filled blastocoel cavity, inner cell mass (ICM), and trophectoderm (TE) [18] [19]. |
This timeline serves as the primary reference for coordinating donor embryo production with host recipient preparation. Deviations from this expected schedule, such as a delay in compaction or blastulation, can indicate reduced embryonic viability and disqualify an embryo from being used as a reliable timed donor.
Protocols for Generating Timed Donor Embryos
Protocol 1: Setting Up Timed Pregnancies
This protocol describes the procedure for mating mice to obtain embryos at a precisely known gestational age [20] [21].
Objective: To generate a cohort of female mice with a known mating time (Day 0.5) for the harvest of aged-matched embryos. Materials:
- Proven adult stud male mice (e.g., C57BL/6, Swiss Webster), 3-4 months old, individually housed.
- Virgin female mice, 8-15 weeks old.
- Standard rodent cages and bedding.
Methodology:
- Pre-conditioning of Males: House stud males individually for 1-2 weeks prior to mating to maximize sperm count and fertility [21].
- Estrous Cycle Synchronization (Optional but Recommended): Group-house female mice (4-10 per cage) for 10-14 days to synchronize their estrous cycles via the Lee-Boot effect. For enhanced synchronization, introduce soiled bedding from a male's cage into the females' cage 48-72 hours before mating to induce the Whitten effect [21].
- Visual Estrous Staging: In the late afternoon prior to mating, select females that are in proestrus or estrus. Indicators include a swollen, pink, and moist vaginal opening [21].
- Timed Mating: In the evening, place 1-2 selected females into a single-housed male's cage.
- Plug Check: Check females for the presence of a vaginal plug early the next morning. The plug is a viscous substance from the male ejaculate that fills the vagina [21].
- Designation of Gestational Day: The morning a vaginal plug is identified is designated as Gestational Day 0.5 (E0.5) or 0.5 days post-coitum (dpc) [21].
Application Notes:
- A vaginal plug confirms copulation but not necessarily pregnancy. Using females in estrus increases the pregnancy-to-plug incidence to 80-90% [21].
- Strains with superior reproductive performance, such as outbred Swiss Webster mice, are preferred for generating large embryo yields. Inbred strains like C57BL/6 are used when genetic uniformity is required but have smaller litter sizes [20].
Protocol 2: In Vitro Fertilization (IVF) and Embryo Culture
For experiments requiring genetic manipulation or precise control over fertilization, IVF is employed.
Objective: To generate embryos in vitro and culture them to specific developmental stages for use as timed donors. Materials:
- Hormones: Pregnant Mare's Serum Gonadotropin (PMSG), Human Chorionic Gonadotropin (hCG).
- M2 and KSOM embryo culture media.
- Mineral oil for embryo culture overlay.
- CO₂ incubator maintained at 37°C, 5% CO₂.
Methodology:
- Superovulation: Administer an intraperitoneal injection of 7.15 IU PMSG to donor female mice, followed by 5 IU hCG 48 hours later [22].
- Oocyte and Sperm Collection: 13-14 hours post-hCG, euthanize females and collect oocytes from the oviductal ampullae. Collect sperm from the cauda epididymis of a stud male [22].
- In Vitro Fertilization: Co-incubate oocytes and capacitated sperm in fertilization medium (e.g., HTF) for 4-6 hours [17] [22].
- Embryo Culture:
- Day 0/1: Approximately 16-18 hours post-insemination, assess fertilization by identifying zygotes with two pronuclei (2PN). Wash and transfer to fresh culture medium [18].
- Days 1-3 (Cleavage Stages): Culture embryos, checking for division to 2-cell, 4-cell, and 8-cell stages. Transfer to sequential medium optimized for later stages if culturing to blastocyst [18].
- Days 4-5 (Morula to Blastocyst): Culture embryos until the morula compact and form blastocysts [18].
Application Notes:
- Supplementing culture medium with oviductal extracellular vesicles (EVs) from specific estrous stages (estrus, metestrus, diestrus) can significantly improve blastocyst yield and quality by better mimicking the in vivo environment [23].
- Only about 50% of fertilized embryos typically develop to the blastocyst stage under standard in vitro conditions [19].
Advanced Assessment and Manipulation of Timed Donors
Non-Invasive Metabolic Assessment
Raman spectroscopy offers a non-invasive method to assess the developmental potential of timed donor embryos by analyzing their metabolic profile.
Principle: This technique quantifies dynamic changes in glucose consumption from the culture medium and maps intracellular distributions of biomolecules like lipids, proteins, and nucleic acids within the embryo [22].
Key Findings:
- Glucose metabolism is stable from E0.5 to E3.5 but increases significantly around the morula-to-blastocyst transition (E4.5), peaking during blastocyst formation [22].
- Embryos that successfully form blastocysts ("encapsulated group") demonstrate a higher glucose metabolic ratio compared to those that arrest ("unencapsulated group") as early as E1.5 [22].
- Raman imaging reveals a high concentration of lipid droplets in the cytoplasm of 1-cell and 2-cell embryos, which are progressively consumed during subsequent development [22].
Application: This metabolic phenotyping can be integrated into the timed donor pipeline to non-invasively select the most viable embryos for transfer or experimental use, thereby increasing experimental efficiency.
The Critical Protocol of Isochronic Cell Injection
The requirement for developmental timing is most rigorously demonstrated in chimera generation experiments.
Objective: To functionally test the developmental competence of donor cells by injecting them into host embryos. Materials:
- Timed donor embryos (e.g., at E3.5 blastocyst stage).
- Donor cells (e.g., Embryonic Stem Cells - ESCs, or Neural Crest Cells - NCCs).
- Microinjection setup.
Methodology and Evidence:
- Isochronic Injection (Matched): Inject pluripotent ESCs into a host blastocyst (E3.5). This results in robust chimera formation with donor cell contribution to all tissues [16].
- Heterochronic Injection (Mismatched):
Conclusion: Successful chimera formation is exclusively dependent on isochronic injection. Donor cells must be developmentally matched to the host embryo's stage to integrate functionally into the developmental program [16]. This principle directly defines the "Timed Donor"—its value is relative to the specific developmental context of the host system.
Diagram: Developmental Match is Crucial for Chimera Formation
The Scientist's Toolkit: Essential Reagent Solutions
Table 2: Key Research Reagents for Timed Donor Embryo Research
| Reagent / Material | Function / Application | Example / Note |
|---|---|---|
| PMSG & hCG | Hormonal regimen for superovulation in mice to obtain a large, synchronized cohort of oocytes [22]. | Typically administered 48 hours apart (e.g., 7.15 IU PMSG, then 5 IU hCG) [22]. |
| KSOM / mHTF Media | Sequential culture media designed to support the changing metabolic needs of the preimplantation mouse embryo from zygote to blastocyst [18] [22]. | |
| Oviductal Extracellular Vesicles (EVs) | Supplement to in vitro culture media to improve embryonic development by providing maternal cues; efficacy is stage-dependent (estrus, metestrus, diestrus) [23]. | Isolated from oviductal fluid; contain cargo (proteins, miRNAs) that support embryo development [23]. |
| Proven Stud Males | Reliable mating partners for setting up timed pregnancies. Strains like Swiss Webster (outbred) or C57BL/6J (inbred) are commonly used [20] [21]. | Isolated housing for 1-2 weeks prior to mating improves success rates [21]. |
| CD-1 or Swiss Webster Foster Females | Pseudopregnant recipient females for embryo transfer following IVF or genetic manipulation. | Vasectomized males are used to induce pseudopregnancy in these females. |
The "Timed Donor" is not merely a chronological concept but a biological standard defined by rigorous developmental synchrony. From the initial setup of timed matings to the final validation of developmental competence through metabolic profiling or functional chimera assays, every step must be calibrated against the intrinsic clock of embryonic development. The protocols and assessment tools outlined here provide a framework for achieving this critical precision, ensuring that donor embryos serve as reliable and reproducible tools in advanced biomedical research. Adherence to these principles of developmental staging is fundamental to the generation of robust, interpretable, and impactful scientific data.
The Scientist's Toolkit: Core Equipment and Reagents
Successful mouse in vitro fertilization (IVF) relies on specialized equipment and rigorously tested reagents to ensure consistent and reproducible results for colony management and timed embryo production.
Core Equipment Essentials
Table 1: Essential Equipment for Mouse IVF Protocols
| Equipment Category | Specific Instrument | Primary Function in Protocol |
|---|---|---|
| Environmental Control | Humidified incubator (37°C, 5% CO₂) [24] | Maintains physiological conditions for gamete co-incubation and embryo culture. |
| Microscopy | Stereomicroscope [24] | Macroscopic procedures: dissecting oviducts, collecting oocytes, and handling embryos. |
| Inverted microscope [24] | Detailed assessment of oocytes, sperm motility, and fertilization status (pronuclei observation). | |
| Sample Handling | Micropipettes & specific pipette tips (e.g., for insemination) [24] | Precise handling and transfer of small fluid volumes, sperm, and embryos. |
| Procedure Consumables | Plastic dishes (e.g., 35mm x 10mm) [24] | Preparation of sperm, fertilization, and embryo washing drops under paraffin oil. |
| Glass capillaries [24] | Gentle handling and transfer of embryos between culture drops. | |
| Surgical Tools | Fine scissors, watchmaker's forceps (#5), micro-spring scissors, dissecting needle [24] | Surgical dissection of reproductive tissues and collection of oocytes and sperm. |
Research Reagent Solutions
Table 2: Key Reagents and Media for Mouse IVF
| Reagent/Media | Function | Examples & Key Components |
|---|---|---|
| Hormones for Superovulation | Stimulate follicle development and trigger ovulation in female donors. | PMSG (Pregnant Mare's Serum Gonadotropin), hCG (human Chorionic Gonadotropin) [25] [24]. |
| Sperm Preincubation Medium | Supports sperm capacitation, a prerequisite for fertilization. | FERTIUP PM (CARD) [24]; c-TYH medium (contains methyl-β-cyclodextrin for cholesterol efflux) [26]. |
| Fertilization Medium | The environment where sperm and oocytes are co-incubated for IVF. | CARD MEDIUM [24]; modified RVF (mRVF) with elevated calcium and glutathione [26]; HTF (Human Tubal Fluid) [26]. |
| Embryo Culture/Washing Medium | Supports development of fertilized embryos to transferable stages. | mHTF [24]; KSOM (Potassium Simplex Optimized Medium) [27]. |
| Cryopreservation Reagents | Protects sperm during freezing and thawing for archiving and recovery. | Cryoprotective Agent (CPA) with raffinose and skim milk, supplemented with L-glutamine (CARD) or monothioglycerol (JAX) [26] [28]. |
Experimental Workflow and Protocols
The mouse IVF process is a multi-stage procedure requiring precise coordination. The following workflow outlines the key steps from preparation to embryo transfer.
Figure 1: Comprehensive workflow for mouse in vitro fertilization (IVF), detailing the sequential steps from donor preparation to embryo transfer.
Detailed Protocol: Superovulation of Donor Females
Superovulation increases the yield of oocytes for fertilization [25].
- Hormone Preparation: Reconstitute PMSG and hCG in sterile saline (e.g., 37.5 IU/mL) [24]. Aliquot and store frozen at -20°C.
- PMSG Injection: Administer an intraperitoneal (IP) injection of PMSG (e.g., 5.0 IU) to 3-5 week-old young female mice during the light cycle, typically between 2:00 PM and 6:00 PM [25] [24]. This stimulates follicular development.
- hCG Injection: Administer an IP injection of hCG (e.g., 5.0 IU) 48 hours after the PMSG injection [25] [24]. This triggers ovulation. Oocytes will be ready for collection 15-17 hours post-hCG.
The quality of sperm directly correlates with fertilization success [25].
- Collection: Sacrifice a mature male mouse (3-6 months old). Isolate the cauda epididymides, minimizing contact with fat and blood. Place the tissue in a drop of pre-equilibrated preincubation medium under mineral oil [24].
- Release Sperm: Puncture the epididymal ducts with fine scissors or a needle and gently squeeze to release the sperm mass [24].
- Capacitation: Transfer the sperm clots into the preincubation drop (e.g., FERTIUP PM or c-TYH). Incubate for 60 minutes in a humidified incubator at 37°C with 5% CO₂ to allow for capacitation. Sperm with high fertility will show vigorous, vortex-like movement [24].
Detailed Protocol: Oocyte Collection and In Vitro Fertilization
- Oocyte Collection: Sacrifice superovulated females 15-17 hours post-hCG. Rapidly dissect and remove the oviducts (ampullae). Using forceps and a needle, tear open the ampulla under oil in a fertilization dish to release the cumulus-oocyte complexes (COCs) into the CARD MEDIUM or mRVF drop [24]. The entire process from sacrifice to COC release should be completed as quickly as possible (within 30 seconds per mouse) [24].
- Insemination: After capacitation, add approximately 3 µL of the sperm suspension to the fertilization drop containing the COCs [24]. Co-incubate the gametes for 3-6 hours in the incubator.
Post-IVF Embryo Handling and Culture
- Washing and Assessment: After 3-6 hours of co-incubation, wash the oocytes several times in fresh washing medium (e.g., mHTF) to remove excess sperm and debris [24].
- Fertilization Check: At around 6 hours post-insemination, observe the oocytes for pronuclei. A fertilized oocyte will have two pronuclei (male and female), while an unfertilized oocyte has none, and a parthenogenetic oocyte has only one [24]. Remove unfertilized and parthenogenetic oocytes.
- Embryo Culture: Culture the presumed fertilized oocytes overnight. Successful fertilization is confirmed by the formation of 2-cell embryos the next day [25] [24]. These 2-cell embryos can then be surgically transferred to pseudo-pregnant recipient females or cultured further to the blastocyst stage in media like KSOM for experimental analysis [27].
Quantitative Data and Protocol Comparisons
Cryopreservation Methodologies
Cryopreservation is a key component of managing genetically engineered mouse model (GEMM) resources, with sperm cryopreservation now often preferred for its efficiency [28].
Table 3: Comparison of Mouse Sperm Cryopreservation Protocols
| Parameter | CARD (Nakagata) Protocol | JAX (Ostermeier) Protocol |
|---|---|---|
| Cryoprotectant Agent (CPA) | L-glutamine [26] [28] | Monothioglycerol (MTG) [26] [28] |
| Sperm Source | Cauda epididymis [28] | Vas deferens and cauda epididymis [28] |
| Preincubation Medium | c-TYH (with methyl-β-cyclodextrin) [26] | HTF or commercial RVF medium [26] |
| Fertilization Medium | HTF with elevated calcium & glutathione [26] | HTF or RVF [26] |
| Typical Scale | 18-20 straws from 2 males [28] | ~25 straws from 2 males [28] |
Quality Control Benchmarks
Rigorous quality control is essential for a reliable cryopreservation and IVF program [25] [28].
Table 4: Standard Quality Control Metrics in Mouse IVF and Cryopreservation
| Process | QC Metric | Passing Standard | Consequence of Failure |
|---|---|---|---|
| Sperm Cryopreservation | Fertilization Rate via IVF | ≥20% 2-cell embryos [25] | Core may repeat at 50% cost [25] |
| Embryo Cryopreservation | Post-thaw Viability Rate | >60% embryo survival [25] | N/A |
| In Vitro Fertilization (IVF) | Pup Birth Rate | Highly variable (10-60+ pups); no pups indicates failure [25] | Core may repeat at 50% cost if more sperm is available [25] |
Advanced culture techniques continue to be explored to further improve embryo development in vitro. A 2025 study demonstrated that supplementing culture media with oviductal extracellular vesicles (EVs) from specific stages of the estrous cycle (estrus, metestrus, diestrus) significantly improved blastocyst yield and hatching rates in mouse IVF embryos [23].
Step-by-Step IVF Protocol for Generating Precisely Timed Embryos
Superovulation is a fundamental technique in reproductive biology and transgenic science, used to artificially induce the release of a large number of oocytes from female mice at a predictable time. This process is a critical first step for in vitro fertilization (IVF), zygote collection for pronuclear injection, and embryo cryopreservation, enabling the efficient generation and preservation of genetically engineered mouse models. The core of the superovulation protocol involves the sequential administration of two gonadotropin hormones: pregnant mare serum gonadotropin (PMSG), which mimics follicle-stimulating hormone (FSH) to stimulate the growth of multiple follicles, and human chorionic gonadotropin (hCG), which mimics luteinizing hormone (LH) to induce final oocyte maturation and ovulation. The precise timing of these injections is paramount to maximizing oocyte yield and quality for subsequent experimental procedures. This application note details established hormonal schedules and provides essential optimization data to frame this technique within the context of a timed IVF protocol for embryo donor mice.
Experimental Protocol: Standard Superovulation Procedure
The following protocol outlines the standard methodology for superovulating mice, as utilized by leading research institutions [29] [30] [31].
Reagents and Materials
- PMSG: Lyophilized powder, reconstituted in sterile PBS or saline.
- hCG: Lyophilized powder, reconstituted in sterile PBS or saline.
- Female Mice: Typically 3-5 weeks old, depending on strain.
- Stud Male Mice: 8 weeks or older, proven breeders.
- 1 ml Syringes and Fine Needles (e.g., 27 G).
- Sterile PBS.
Step-by-Step Procedure
- Hormone Preparation: Reconstitute PMSG and hCG according to manufacturer instructions. Aliquot and store frozen at -20°C or -80°C. Before injection, dilute aliquots to a concentration of 50 IU/ml to deliver a 5 IU dose in a 0.1 ml volume [29] [31].
- PMSG Injection: On Day 1, intraperitoneally (IP) inject each female mouse with 5 IU of PMSG (0.1 ml of a 50 IU/ml solution). This is typically performed in the afternoon, between 1:00 PM and 4:00 PM [29] [30].
- hCG Injection: After precisely 42 to 52 hours (typically on Day 3), IP inject each female with 5 IU of hCG (0.1 ml of a 50 IU/ml solution) [30] [13].
- Mating: Immediately following the hCG injection, place each superovulated female into a cage with a single stud male mouse [29].
- Oviduct and Oocyte/Embryo Collection:
- For zygote collection (0.5 days post coitum, dpc), check for a vaginal copulatory plug the next morning (Day 4). Harvest zygotes from the oviducts [29].
- For morula collection (2.5 dpc), harvest embryos from the oviducts or uterus [29].
- For blastocyst collection (3.5 dpc), flush embryos from the uterus [31].
The following workflow diagram illustrates the sequence and timing of these key steps.
Strain-Specific Optimization of Hormone Schedules
The standard 5 IU PMSG/5 IU hCG protocol does not yield optimal results for all mouse strains. Genetic background significantly influences the superovulation response, necessitating adjustments to hormone dosage and the age or weight of the donor female [32]. The table below summarizes optimized parameters for several commonly used inbred and hybrid strains.
Table 1: Strain-Specific Optimization for Superovulation
| Mouse Strain | Optimal Female Weight | Optimal PMSG Dose | Optimal Protocol Notes | Expected Oocyte Yield |
|---|---|---|---|---|
| C57BL/6 | 10.5 - 14.4 g [32] | 5 IU [32] | Younger, lighter females within this range respond best. Two doses of 5 IU PMSG one week apart may increase yield [32]. | Good [32] |
| FVB/N | 14.5 - 16.4 g [32] | 5 IU [32] | Best response in this weight range, though oocyte yield can be variable [32]. | Variable [32] |
| BALB/c | ≤ 14.8 g [32] | 5 IU [32] | Best response in younger, lighter females [32]. | Good [32] |
| B6D2F1 | 6.0 - 9.9 g [32] | 5 IU [32] | This hybrid strain responds well at a very young age and low weight [32]. | Excellent [32] |
| B6(Cg)-Tyrc-2J/J | ≤ 13.7 g [32] | 2.5 IU [32] | A lower PMSG dose (2.5 IU) produces more oocytes than the standard 5 IU dose [32]. | Improved with lower dose [32] |
| CD-1 (ICR) | ≥ 23.5 g [32] | 5 IU [32] | Unlike most strains, older, heavier females yield a better superovulation response [32]. | Good [32] |
Integrated Application in an IVF Workflow
Superovulation is the initial and most critical step in a multi-day IVF and embryo culture workflow. The timing of hormone injections dictates the schedule for all subsequent steps, from sperm preparation to embryo transfer. The following diagram integrates the superovulation schedule into a complete, timed IVF protocol, such as the CARD (Center for Animal Resources and Development) method [13].
The Scientist's Toolkit: Essential Reagents and Materials
Successful superovulation and subsequent IVF rely on high-quality, specific reagents. The following table lists key materials and their functions in the protocol.
Table 2: Essential Research Reagents and Materials
| Item | Function / Application in Protocol |
|---|---|
| PMSG (e.g., Sigma G4877) | A gonadotropin with FSH-like activity; stimulates the synchronous development of a large cohort of ovarian follicles [29] [30]. |
| hCG (e.g., Sigma C1063) | A gonadotropin with LH-like activity; triggers final oocyte maturation and ovulation approximately 12-15 hours after administration [29] [30]. |
| FERTIUP Medium | Sperm pre-incubation medium used for sperm capacitation for 30-60 minutes prior to IVF [13]. |
| CARD MEDIUM | Specialized fertilization medium; cumulus-oocyte complexes (COCs) are incubated here prior to and during insemination [13]. |
| mHTF Medium | Modified Human Tubal Fluid medium; used for washing and culturing embryos after fertilization [13]. |
| Mineral Oil (Embryo-tested) | Used to overlay microdrop cultures of media to prevent evaporation and maintain pH and osmolarity [33] [13]. |
| IBMX (3-Isobutyl-1-methylxanthine) | A phosphodiesterase inhibitor used in oocyte collection medium to maintain meiotic arrest at the Germinal Vesicle (GV) stage for synchronized in vitro maturation [33]. |
The hormonal superovulation schedule using PMSG and hCG is a well-established but finely tunable technique. The consistent success of this procedure in generating high-quality, developmentally competent oocytes and embryos hinges on three principal factors: the precise timing of hormone injections to mimic the natural estrous cycle, the adaptation of the protocol to the genetic background of the mouse strain, and the integration of this schedule with downstream IVF or embryo collection steps.
As detailed in the protocols and data above, a one-size-fits-all approach is suboptimal. Researchers must empirically determine the best combination of female age/weight and hormone dose for their specific mouse strain to maximize oocyte yield and minimize the number of animals used, in accordance with the principles of the 3Rs (Replacement, Reduction, and Refinement) [32]. When optimized and seamlessly integrated into a comprehensive IVF workflow—from sperm preparation to embryo culture and transfer—the superovulation protocol becomes a powerful and reproducible tool for the efficient production of timed embryos, ensuring the success of advanced research in genetics, development, and drug discovery.
This document provides detailed application notes and protocols for sperm collection and preparation, framed within the context of In vitro fertilization (IVF) protocol for timed embryo donor mice research. The procedures outlined are critical for generating genetically engineered mouse models and for reproductive toxicology studies. Standardized and efficient sperm handling techniques directly impact key research metrics, including fertilization rates, embryo quality, and the overall success of transgenic mouse line propagation. The following sections provide a comprehensive guide to the core methodologies for collecting and processing both fresh and frozen sperm samples, complete with quantitative comparisons and detailed workflows.
Sperm Collection Techniques
The method of sperm collection is chosen based on the experimental design, the desired genetic outcome, and the availability of donor males.
Masturbation and Ejaculate Collection
This is the standard method for obtaining sperm from larger animal models. For murine models, this is less common but can be adapted.
- Key Protocol Steps:
- Abstinence Period: Observe a period of abstinence (no ejaculation) for at least two days but not more than five days before collection to optimize sperm quantity and quality [34].
- Collection Container: Collect semen in a sterile, non-toxic plastic specimen cup provided by the laboratory. Commercial condoms are not acceptable as they are toxic to sperm [34].
- Handling and Transport: Wash and dry hands prior to collection. Do not use lubricants unless specifically directed by a protocol. If collected at home, minimize transit time and transport the sample at room or body temperature [34].
Surgical Sperm Retrieval
For mice, sperm is typically collected post-sacrifice from the epididymides. In azoospermic (no sperm in ejaculate) research models, surgical retrieval is necessary.
Epididymal Sperm Collection (for Mice):
- Euthanize the donor male mouse following approved animal welfare protocols.
- Excise the cauda epididymides (the tail of the epididymis) and place them in a pre-warmed culture medium.
- Puncture or mince the epididymal tissue with fine needles or a scalpel blade to allow sperm to swim out into the medium [35].
- Assess the sperm number and motility under a microscope. If insufficient, the IVF process may be cancelled [35].
Microdissection Testicular Sperm Extraction (Micro-TESE): This is a sophisticated surgical technique used in azoospermic models where sperm production is impaired. It is the gold standard for sperm retrieval in cases of non-obstructive azoospermia (NOA) [36].
- The procedure is performed under general anesthesia with the aid of an operating surgical microscope.
- A midline incision is made in the testis to expose the parenchyma.
- The seminiferous tubules are examined; more dilated, opaque tubules are selected for extraction as they are more likely to contain sperm [36].
- The retrieved tissue is processed by the embryology lab. Technicians extract sperm cells from the tubules using fine needles or glass slides, sometimes employing enzymatic digestion with collagenase to facilitate extraction [36].
Table 1: Comparison of Sperm Retrieval Techniques in a Research Context
| Technique | Primary Application | Key Advantage | Key Disadvantage | Sperm Retrieval Rate (Approx.) |
|---|---|---|---|---|
| Masturbation/Ejaculate | Non-surgical collection from capable subjects | Non-invasive; high yield from fertile males | Not applicable to murine models or azoospermic subjects | N/A |
| Epididymal Collection | Standard murine IVF; obstructive azoospermia | High yield of mature, motile sperm from mice | Requires euthanasia of the donor male [35] | Dependent on male fertility |
| Micro-TESE | Non-obstructive azoospermia (NOA) models | Highest retrieval rate for NOA; minimal tissue removal [36] | Requires specialized microsurgical expertise and longer operating time [37] | 40-60% [36] |
| Testicular Sperm Aspiration (TESA) | Obstructive azoospermia models | Simple, cost-effective needle aspiration [37] | Lower retrieval rate compared to Micro-TESE; may not provide enough tissue [37] | Lower than Micro-TESE |
Sperm Collection Workflow
The following diagram illustrates the decision pathway for selecting an appropriate sperm collection method based on the research model and objectives.
Sperm Preparation and Processing Techniques
Sperm preparation techniques are designed to select a population of motile, morphologically normal, and viable spermatozoa while removing seminal plasma, dead sperm, and other contaminants.
Standard Preparation Methods
Swim-Up Technique: This method leverages the innate motility of healthy sperm. The sperm sample is placed under a layer of culture medium. Motile sperm swim up into the medium, which is then collected.
- Mix the sperm sample with a washing medium volume-to-volume (v/v) and centrifuge (e.g., 320g for 10 minutes) [38].
- Discard the supernatant and gently layer culture medium over the pellet.
- Incubate the tube at a 45-degree angle for 30-60 minutes in a CO₂ incubator.
- Carefully harvest the top medium layer, which is now enriched with motile sperm [38].
Density Gradient Centrifugation (DGC): This technique uses a colloidal silica gradient to separate sperm based on their buoyancy and motility. Mature, morphologically normal sperm with intact DNA penetrate further into the gradient.
Advanced Sperm Sorting
- Microfluidic Sperm Sorting: This technology uses microfluidic chips to isolate progressive motile sperm directly from the ejaculate based on their swimming ability and hydrodynamic properties. A study comparing it to the swim-up technique found significantly higher sperm concentration, progressive motility levels, and fertilization rates in the microfluidic group [39].
Preparation Technique Outcomes
Table 2: Quantitative Comparison of Sperm Preparation Techniques
| Technique | Sperm Concentration Recovery | Progressive Motility Recovery | Fertilization Rate | Key Application |
|---|---|---|---|---|
| Swim-Up | Baseline | Baseline | Baseline | Good for normozoospermic samples; common standard [38] |
| Density Gradient Centrifugation | Higher than Swim-Up | Higher than Swim-Up | Comparable to Swim-Up | Effective for samples with debris and dead sperm [38] |
| Microfluidic Sorting | Significantly Higher [39] | Significantly Higher [39] | Significantly Higher [39] | Emerging technology for superior motile sperm selection |
Cryopreservation of Sperm
Sperm cryopreservation, or sperm banking, is a fundamental technique for preserving valuable genetic material from transgenic mouse lines for future IVF cycles or distribution.
Cryopreservation Protocols
Conventional Slow Freezing:
- The sperm sample is mixed with a cryoprotectant solution (e.g., containing glycerol) to protect the cells from ice crystal formation [40] [36].
- The mixture is loaded into straws or vials.
- Using a programmable freezer, the sample is cooled at a controlled, slow rate (e.g., -10°C/min) [36].
- The frozen samples are plunged into liquid nitrogen for long-term storage at -196°C [40].
Vitrification: This method uses ultrarapid cooling to transform cellular water into a glass-like state without forming ice crystals.
- Sperm are suspended in a higher concentration of cryoprotectants.
- A very small volume of the sample is directly plunged into liquid nitrogen, achieving extremely high cooling rates [36].
- While promising, the World Health Organization still considers sperm vitrification an experimental technique [36].
Pre-freeze Processing: A Critical Factor
Research indicates that the timing of sperm preparation relative to freezing significantly impacts post-thaw quality.
- Freezing BEFORE Swim-up (Recommended): Cryopreserving the whole ejaculate prior to sperm selection leads to higher total and progressive motility, total motile sperm count, and viability rates after thawing. The seminal plasma has a protective role during the freezing process, containing antioxidants that prevent reactive oxygen species (ROS)-induced damage [38].
- Freezing AFTER Swim-up: Performing sperm selection prior to freezing reaches critical values, especially when subfertile patients or models are considered, leading to poorer post-thaw outcomes [38].
Fresh vs. Frozen Sperm in ART
The choice between using fresh or frozen sperm involves a trade-off between sperm quality and logistical flexibility.
Table 3: Comparison of Fresh vs. Frozen Sperm Use in ART Cycles
| Parameter | Fresh Sperm | Frozen Sperm |
|---|---|---|
| Sperm Quality | Generally higher quality metrics (motility, viability) [36] | Some quality loss due to cryodamage; vitrification improves outcomes [36] |
| Logistical Flexibility | Requires perfect timing with oocyte retrieval | Decouples sperm retrieval from IVF cycle; available as backup [40] [36] |
| Fertilization Rate | Traditionally higher | Comparable rates in many studies [41] [36] |
| Pregnancy/Live Birth Rate | Slightly higher in some studies | Comparable to fresh in many cases, especially with donor sperm [41] [36] |
| Application in Mouse Research | Used when a male is euthanized for immediate IVF [35] | Essential for preserving and distributing valuable genetic lines [35] |
The Scientist's Toolkit: Essential Reagents and Materials
Table 4: Key Research Reagent Solutions for Sperm Collection and Preparation
| Reagent/Material | Function | Example |
|---|---|---|
| Cryoprotectant Agents | Protect sperm from ice crystal damage during freezing/thawing by replacing intracellular water. | Glycerol, Ethylene Glycol, sucrose, trehalose [40] [36] |
| Holding/Culture Media | Provide a physiological environment for sperm during collection, processing, and incubation. | Human Tubal Fluid (HTF), Flushing Medium, IVF Medium [38] |
| Density Gradient Medium | Form a density barrier for centrifugation-based selection of motile, morphologically normal sperm. | Colloidal Silica Suspensions |
| Hypoosmotic Swelling Test (HOS) Solution | Assess sperm membrane integrity and viability; viable sperm with intact membranes exhibit tail swelling. | Solution of sodium citrate and fructose [38] |
| Enzymatic Digestion Agents | Facilitate the extraction of sperm from dense testicular tissue in surgical retrievals. | Collagenase [36] |
| Liquid Nitrogen | Provides ultra-low temperature environment (-196°C) for long-term cryostorage of sperm samples. | N/A [40] |
Integrated Experimental Workflow
The following diagram summarizes the complete integrated workflow for sperm collection, processing, cryopreservation, and use in IVF, contextualized for research settings.
Oocyte Collection and Cumulus Complex Isolation from Donor Females
The isolation of high-quality cumulus-oocyte complexes (COCs) from donor females is a critical foundational step in mouse in vitro fertilization (IVF) research. This procedure enables the generation of timed embryos for studying development, genetic modification, and colony management. Efficiency in oocyte collection and the subsequent removal of cumulus cells are paramount for achieving high fertilization and blastocyst formation rates. This application note details a standardized, reliable protocol for oocyte collection and COC isolation, incorporating both established methods and emerging technologies to enhance reproducibility and success in a research setting.
Materials and Reagents
Research Reagent Solutions
The following table lists the essential reagents and materials required for the successful execution of oocyte collection and cumulus complex isolation.
Table 1: Key Research Reagents and Materials for Oocyte Collection and Cumulus Complex Isolation
| Item | Function/Application | Examples/Specifications |
|---|---|---|
| Pregnant Mare Serum Gonadotropin (PMSG) | Hormonal priming to stimulate follicle growth and superovulation [42] [14]. | Typically 5-10 IU administered via intraperitoneal injection [42] [13]. |
| Human Chorionic Gonadotropin (hCG) | Triggers final oocyte maturation and ovulation [42] [14]. | 5-10 IU injected 46-52 hours after PMSG [42] [13]. |
| Hyaluronidase | Enzyme that digests hyaluronic acid matrix, loosening cumulus cells for removal [42] [43]. | Concentration and exposure time must be optimized (e.g., 30-120 seconds) [43]. |
| Handling Media (e.g., M2, HTF-HEPES) | Maintains pH and osmotic balance during oocyte collection and manipulation outside the incubator [42] [14]. | Used for dissecting oviducts and collecting COCs. |
| Fertilization/Culture Media (e.g., CARD, HTF, KSOM-AA) | Supports sperm capacitation, fertilization, and subsequent embryo development [13] [14]. | Pre-equilibrated in a CO₂ incubator prior to use. |
| Mineral Oil (Embryo-tested) | Overlays culture media drops to prevent evaporation and osmolarity shifts [13] [14]. | Light mineral oil, pre-gassed. |
| Wide-Bore Pipettes/Tips | For handling COCs and denuded oocytes without causing mechanical damage to the zona pellucida [13] [14]. | Essential for manual pipetting steps. |
Experimental Workflow and Protocol
The overall process from preparing donor mice to obtaining isolated oocytes ready for IVF is a multi-day procedure. The following workflow diagram outlines the key stages and their temporal relationships.
Superovulation of Donor Females
The process begins with the hormonal stimulation of prepubertal or young adult female mice (typically 4-6 weeks old) to maximize oocyte yield [42] [35].
- Day 1: Administer an intraperitoneal injection of 5-10 IU of PMSG to stimulate follicular development [42] [13] [14].
- Day 3: Approximately 46-52 hours after the PMSG injection, administer an intraperitoneal injection of 5-10 IU of hCG to trigger final oocyte maturation and ovulation [42] [13]. Oocyte collection is performed 13-17 hours post-hCG injection [14].
Oocyte Collection and Cumulus-Oocyte Complex (COC) Isolation
The following protocol details the steps for collecting COCs after the administration of hCG.
- Sacrifice and Dissection: At 13-17 hours post-hCG injection, euthanize the donor females according to approved institutional protocols [13]. Quickly dissect to expose the reproductive tract. Remove the oviducts, trimming away excess fat and connective tissue.
- COC Release: Place the oviducts into a dish containing pre-equilibrated handling medium (e.g., M2 or HTF-HEPES) under oil. Using fine forceps (#5), carefully tear open the swollen ampulla of the oviduct to release the dense, cloud-like cumulus mass containing the oocytes [13] [14].
- COC Harvesting: Using a wide-bore pipette tip to prevent damage, transfer the cumulus masses from multiple females into a pre-equilibrated fertilization dish (e.g., containing CARD medium or HTF) [13]. Incubate the collected COCs for 30-60 minutes before proceeding with cumulus removal [13].
Cumulus Cell Removal
Cumulus removal (CR) is essential for assessing oocyte maturity prior to Intracytoplasmic Sperm Injection (ICSI) and for preventing genetic contamination in subsequent preimplantation genetic testing [43] [44]. The following methods are commonly employed.
Table 2: Methods for Cumulus Cell Removal
| Method | Protocol | Considerations |
|---|---|---|
| Enzymatic + Mechanical | Incubate COCs in a hyaluronidase solution (concentration and time as per manufacturer's guidelines, typically 30-120 sec) to dissolve the hyaluronic acid matrix. Subsequently, pipet the COCs gently with a wide-bore pipette to dislodge the loosened cumulus cells [42] [43]. Wash oocytes thoroughly to remove enzyme and cellular debris. | Standard and widely used. Requires careful timing to avoid oocyte exposure to the enzyme. |
| Mechanical Pipetting | Repeatedly aspirate and dispense the COCs using a narrow-bore pipette (manually or via a robotic system) in a medium without enzyme [44]. The shear force physically strips away the cumulus cells. | Avoids potential chemical stress from enzymes. Requires skill to prevent oocyte loss or damage. |
| Advanced Platforms (Vibration/Robotic) | Vibration-Induced Flow (VIF): Uses an open-surface microfluidic chip with micropillars. Applied vibration creates a flow that selectively removes smaller cumulus cells, leaving oocytes in the loading chamber. Achieves ~99% cell removal efficiency without enzymes [43].Robotic Systems: Employ machine vision to assess oocyte maturity and adaptive control to position COCs accurately during aspiration, achieving high success (98%) and low discard rates [44]. | Promising for standardization and reduced manual labor. Requires specialized equipment. |
Quality Assessment and Troubleshooting
Post-collection assessment is critical for ensuring only high-quality oocytes are used for IVF.
- Oocyte Morphology: Exclude oocytes with very poor morphology, such as dark cytoplasm or fragmented structure [42].
- Embryo Source Quality: If the mice produce fewer than 10 embryos or if the fertilization rate in a group is below 40%, the entire batch may be deemed non-representative due to potential issues with hormone injection or media, and should be excluded from experimental data [42].
- Quantitative Assessment: The use of improved YOLOv5s networks has been demonstrated to quantitatively assess the completeness of cumulus cell removal, ensuring minimal contamination [44].
The reliable collection of oocytes and isolation of cumulus complexes is a technically demanding but essential skill for mouse IVF research. Adherence to a standardized protocol for superovulation, dissection, and cumulus removal, as detailed herein, ensures the consistent yield of high-quality oocytes. The integration of emerging automated technologies for cumulus removal holds significant promise for enhancing reproducibility, throughput, and success rates in generating timed embryos for downstream applications.
In vitro fertilization (IVF) is a cornerstone technique in reproductive biology and genetic engineering, particularly in the creation of genetically modified mouse models for research. The stages of insemination and oocyte co-incubation are critical, as the conditions during these phases directly impact fertilization success, embryonic development, and the viability of resulting embryos. This application note provides a detailed protocol and data analysis for these stages, contextualized within a broader thesis on IVF protocols for timed embryo donor mice. It is designed to support researchers, scientists, and drug development professionals in optimizing laboratory procedures.
Impact of Co-incubation Time on IVF Outcomes
A meta-analysis of randomized controlled trials provides a direct comparison of outcomes between brief (1-6 hours) and long (16-24 hours) gamete co-incubation times [45]. The data below summarize the combined odds ratios (OR) and 95% confidence intervals (CI) for key reproductive outcomes, favoring brief co-incubation when the OR is greater than 1.
Table 1: Outcomes of Brief vs. Long Gamete Co-incubation [45]
| Outcome Measure | Odds Ratio (OR) | 95% Confidence Interval | Statistical Significance |
|---|---|---|---|
| Implantation Rate | 1.97 | 1.52 – 2.57 | Significant |
| Ongoing Pregnancy Rate | 2.18 | 1.44 – 3.29 | Significant |
| Top-Quality Embryo Rate | 1.17 | 1.02 – 1.35 | Significant |
| Live-Birth Rate | 1.09 | 0.72 – 1.65 | Not Significant |
| Clinical Pregnancy Rate | 1.36 | 0.99 – 1.87 | Not Significant |
| Miscarriage Rate | 1.32 | 0.55 – 3.18 | Not Significant |
| Polyspermy Rate | 0.80 | 0.48 – 1.33 | Not Significant |
| Normal Fertilization Rate | 0.89 | 0.80 – 0.99 | Significant (Lower) |
Genetic and Developmental Outcomes of IVF in Mice
Recent research in mouse models highlights the importance of protocol optimization. The data below summarize key findings on genetic integrity and the impact of a modified warming protocol (MWP) for vitrified oocytes.
Table 2: Genetic and Developmental Outcomes in Mouse and Donor Oocyte IVF
| Parameter | Finding | Notes and Context |
|---|---|---|
| New Single-Nucleotide Variants | ≈30% increase in IVF-conceived pups [4] | Compared to natural conception; mutations are spread across the genome and are largely neutral [4]. |
| Absolute Risk of Harmful Mutation | ≈1 additional harmful change per 50 IVF mice [4] | Risk considered "almost negligible" due to genome size [4]. |
| Usable Blastocyst Formation (MWP) | 51.4% [46] | MWP group performed similarly to fresh oocytes (48.5%) and better than conventional warming (35.4%) [46]. |
| Ongoing Pregnancy/Live Birth (MWP) | 66.7% [46] | MWP group showed a significant improvement over the conventional warming protocol (50.4%) [46]. |
Experimental Protocols
Workflow for Mouse IVF and Co-incubation
The following diagram illustrates the core workflow for a mouse IVF procedure, from superovulation to embryo culture.
Detailed Protocol: Insemination and Co-incubation for Mouse IVF
This protocol synthesizes methodologies from established sources [13] [14].
I. Pre-insemination Preparation
- Fertilization Dish Preparation: On the afternoon prior to IVF, prepare the fertilization dish. For every 4-6 superovulated females, place a central 200 µL drop of pre-equilibrated fertilization medium (e.g., CARD medium or HTF) in a culture dish. Cover the drop with pre-gassed, embryo-tested light mineral oil and place the dish in the incubator (37°C, 5% CO₂) at least 10 minutes before the first oocyte collection [13].
- Sperm Preparation:
- Fresh Sperm: Sacrifice a male mouse. Dissect out the cauda epididymides and vasa deferentia, removing excess fat. Place them in a 1 mL drop of sperm preincubation medium (e.g., FERTIUP or HTF) under oil. Mince the tissue with a needle to release sperm and incubate the dish for 30-60 minutes at 37°C, 5% CO₂ for capacitation [13] [14].
- Frozen/Sperm: Thaw a cryovial in a 37°C water bath. For some protocols, the thawed sperm can be expelled directly into a pre-warmed drop of FERTIUP and incubated for 30 minutes before use [13]. Alternatively, the sperm can be washed via centrifugation (735g for 4 minutes) and resuspended in HTF before insemination [14]. Assess sperm motility and concentration.
II. Oocyte Collection and Insemination
- Collect cumulus-oocyte complexes (COCs) from superovulated females approximately 13 hours post-hCG injection. Place the torn ampullae in the oil of the fertilization dish and release the COCs directly into the pre-equilibrated fertilization medium drop. Return the dish to the incubator for 30-60 minutes [13].
- Insemination: After capacitation, add a 3-10 µL aliquot of the motile sperm suspension to the fertilization dish containing the COCs. Use wide-bore pipette tips to avoid damaging gametes. The final motile sperm concentration in the drop should be between 1x10⁶ to 2.5x10⁶ sperm/mL [13] [14].
III. Co-incubation and Post-Incubation Handling
- Co-incubation: Incubate the gametes together for 3-6 hours at 37°C, 5% CO₂ [45] [14]. The brief co-incubation duration helps reduce exposure to reactive oxygen species (ROS) produced by sperm, which can damage oocytes and impair embryo development [45].
- Washing and Culture: After the co-incubation period, wash the oocytes through several drops (e.g., 3-5 drops) of fresh medium (e.g., HTF or mHTF) to remove excess sperm and cellular debris [13] [14].
- Transfer the washed oocytes to a culture dish with pre-equilibrated KSOM-AA medium for overnight culture.
- Fertilization Check: The following morning, assess oocytes for successful fertilization, indicated by the formation of two-cell embryos.
The Scientist's Toolkit
Table 3: Essential Research Reagents and Materials for Mouse IVF
| Reagent/Material | Function/Application | Example Product/Catalog |
|---|---|---|
| PMSG (Pregnant Mare Serum Gonadotropin) | Hormone for superovulation; stimulates follicular development. | - |
| hCG (Human Chorionic Gonadotropin) | Hormone to trigger ovulation; administered after PMSG. | - |
| Fertilization Medium (e.g., HTF, CARD) | Supports the process of fertilization during co-incubation. | CARD MEDIUM (Cosmo Bio, Cat# KYD-003-EX) [13] |
| Sperm Preincubation Medium (e.g., FERTIUP) | Medium used for sperm capacitation before insemination. | FERTIUP (Cosmo Bio, Cat# KYD-002-EX) [13] |
| Culture Medium (KSOM-AA) | Optimized medium for supporting embryonic development post-fertilization. | - |
| Mineral Oil (Embryo-Tested) | Light, sterile oil to overlay culture drops, preventing evaporation and pH shifts. | Sigma (Cat# M8410, M5310) [13] [14] |
| Wide-Bore Pipette Tips | For handling delicate oocytes and embryos without causing shear stress. | Rainin (HR-250W, 1000W) [14] |
This application note provides a detailed protocol for the post-fertilization culture of mouse embryos, specifically from the zygote stage to the 2-cell embryo stage, for use in timed embryo donor research. The period immediately following in vitro fertilization (IVF) is critical, as the embryonic genome begins to activate, and environmental stressors can significantly impact developmental potential. This document outlines standardized methodologies to maximize embryo viability and ensure reproducible results for research and drug development applications. The protocols are framed within the context of generating precisely timed embryos for transfer into pseudopregnant recipient females, a cornerstone technique in reproductive biology and genetic engineering.
Key Developmental Parameters & Culture Media Composition
Successful culture requires careful monitoring of specific morphokinetic parameters and the use of defined media sequences. The table below summarizes the key benchmarks for successful development during this critical window.
Table 1: Key Developmental Parameters from Zygote to 2-Cell Embryo
| Developmental Stage | Approximate Time Post-Insemination | Key Morphological Landmarks | Culture Medium |
|---|---|---|---|
| Zygote | 6 hours | Presence of two pronuclei (male and female) [24] | CARD MEDIUM or specialized fertilization medium [24] |
| Cleavage to 2-Cell | 24-48 hours [47] | Division into two distinct, symmetrical blastomeres [48] | mHTF or Potassium Simplex Optimized Medium [24] [49] |
| Exclusion of Parthenogenetic Oocytes | 6 hours post-insemination | Identification and removal of oocytes with only one pronucleus [24] | mHTF [24] |
The composition of the culture medium is a critical variable. Different media can selectively influence embryo development. For instance, studies have shown that culture media can affect the developmental speed of male and female embryos, potentially leading to a skewed sex ratio at birth, underscoring the medium's biochemical impact on the embryo [50].
Table 2: Common Culture Media and Their Applications
| Media Name | Primary Function | Key Components/Notes |
|---|---|---|
| CARD MEDIUM | Fertilization and initial pre-incubation | Used for insemination and initial culture post-IVF; preparation varies for fresh, frozen, or transported sperm [24] |
| mHTF | Washing and post-fertilization culture | Used for washing oocytes post-insemination and subsequent culture of embryos [24] |
| Potassium Simplex Optimized Medium | In vitro culture to blastocyst stage | Used for culturing 1-celled and 2-celled embryos to blastocysts [49] |
| Sage Media | Commercial culture medium | Associated with faster male embryo development and a higher likelihood of male live births compared to G-TL media [50] |
Detailed Experimental Protocols
Protocol 1: Post-IVF Processing and Identification of Fertilized Zygotes
This protocol begins immediately after the insemination period.
Materials:
- Fertilization dish with oocytes in CARD MEDIUM (from the IVF procedure) [24]
- Prepared washing dish with 4 drops of 80μL mHTF, covered with liquid paraffin [24]
- Pipette tips for handling oocytes/embryos
- Humidified incubator (37°C, 5% CO₂ in air) [24]
Methodology:
- Initial Wash: At 3 hours post-insemination, transfer the cumulus-oocyte-complexes (COCs) from the CARD MEDIUM drop to the first drop of mHTF in the washing dish. Gently pipette to wash the COCs, aiding in the removal of excess sperm and cellular debris [24].
- Subsequent Washes: Move the COCs sequentially through the second and third drops of mHTF for further washing [24].
- Fertilization Check: At 6 hours post-insemination, observe the oocytes in the third mHTF drop under a microscope. The key is to distinguish between fertilized and parthenogenetic oocytes at this stage [24].
- Removal of Non-Fertilized Oocytes: Using a fine pipette, identify and remove all parthenogenetic and unfertilized oocytes. This step is critical, as parthenogenetic oocytes can develop to a 2-cell stage, making them impossible to distinguish from fertilized embryos later [24].
- Overnight Culture: Return the dish with the confirmed zygotes to the incubator for overnight culture [24].
Protocol 2: Culture to 2-Cell Stage and Embryo Selection
This protocol covers the culture period leading to the first cleavage division.
Materials:
- Culture medium (e.g., mHTF or Potassium Simplex Optimized Medium) [24] [49]
- Liquid paraffin
- 35mm plastic culture dishes
- Humidified incubator (37°C, 5% CO₂ in air)
Methodology:
- Morning Assessment: The morning after the fertilization check (approximately 24 hours post-insemination), examine the zygotes. A significant proportion should have undergone cleavage to the 2-cell stage [47].
- Embryo Evaluation: Assess the 2-cell embryos based on morphology. Ideal embryos have symmetrical blastomeres with minimal or no fragmentation [48].
- Transfer and Preparation for Transfer: Select only the normal, healthy 2-cell embryos.
- For immediate embryo transfer, these embryos can be loaded into a transfer pipette for surgical transfer into the oviduct of a pseudopregnant recipient female [51].
- For vitrification, these embryos can be prepared for cryopreservation at the 2-cell stage [24].
- For continued culture, transfer the 2-cell embryos to a fresh drop of medium (e.g., a fourth drop of mHTF) if the experimental goal is to culture them to the blastocyst stage [24].
The Scientist's Toolkit: Essential Research Reagents
The following table details key reagents and their critical functions in the post-fertilization culture workflow.
Table 3: Essential Research Reagent Solutions for Post-Fertilization Culture
| Reagent/Material | Function/Application | Technical Notes |
|---|---|---|
| mHTF Medium | A modified human tubal fluid medium used for washing fertilized oocytes and culturing embryos to the 2-cell stage. | Serves as a base culture medium; requires equilibration in a CO₂ incubator before use [24] |
| CARD MEDIUM | A specialized medium optimized for the fertilization step and the initial pre-incubation of oocytes. | Formulation differs depending on the type of sperm used (fresh, frozen, transported) [24] |
| Potassium Simplex Optimized Medium | A chemically defined medium used for the in vitro culture of 1-celled and 2-celled embryos to the blastocyst stage. | Supports development through key preimplantation stages; should be prepared fresh [49] |
| Liquid Paraffin | Used to overlay microdrop cultures of medium to prevent evaporation and minimize changes in pH and osmolarity. | Essential for maintaining a stable microenvironment for embryo development [24] |
| M2 Medium | A HEPES-buffered medium used for procedures performed outside the incubator at room temperature, such as handling embryos during transfers. | Maintains physiological pH outside of a CO₂ environment [49] |
Workflow Visualization & Embryo Assessment
The following diagram illustrates the complete experimental workflow from the end of insemination to the 2-cell embryo stage, highlighting key decision points and quality control checks.
Figure 1: Experimental workflow for post-fertilization culture from zygote to 2-cell embryo, showing key quality control steps.
A critical component of the protocol is the accurate morphological assessment of embryos at each stage. The diagram below details the decision logic for evaluating and selecting embryos based on strict morphological criteria.
Figure 2: Embryo assessment logic for quality control and selection at key time points.
Troubleshooting Common IVF Failures and Advanced Optimization Strategies
Within the context of generating timed embryos from donor mice for research, consistent and high fertilization rates are critical for experimental reproducibility and efficiency. Low fertilization presents a significant bottleneck, primarily stemming from two sources: deficits in sperm quality and in oocyte maturity. This application note provides detailed protocols and quantitative frameworks for researchers to systematically diagnose and address these issues, thereby enhancing the reliability of in vitro fertilization (IVF) outcomes in mouse models.
Quantitative Assessment of Fertilization Failure
A retrospective clinical study analyzing over 13,000 cycles found that poor fertilization (≤30%) occurred in 3.7% of cycles. Crucially, in cycles where the same donor oocytes were used with different sperm sources, 84.1% of poor fertilization cases were attributed to sperm-related factors, while only 15.9% were linked to non-sperm factors such as the oocyte or laboratory procedures [52]. This underscores the paramount importance of a rigorous sperm investigation. The table below summarizes key quantitative indicators from clinical and experimental studies.
Table 1: Key Parameters Linked to Fertilization Success
| Parameter | Association with Fertilization Outcome | Reference/Model |
|---|---|---|
| Sperm-Related Factors | ||
| PLCζ Mutation/Absence | Leads to failure of oocyte activation and fertilization failure. | [53] |
| Standard Semen Analysis | Normal in 95.5% of poor fertilization cases; poor predictor alone. | [52] |
| Oocyte-Related Factors | ||
| Oocyte Collection Time | Collection >3 min post-euthanasia reduces fertilization rates in rats. | [15] |
| Number of Mature Oocytes (MII) | Significantly higher in cycles resulting in embryo transfer after prior failure. | [54] |
| Combined Indicators | ||
| Follicle Size (on hCG day) | Larger follicles (e.g., 15-17mm) associated with higher oocyte maturity. | [54] |
| Estradiol Level (on hCG day) | Significantly higher in successful cycles versus total fertilization failure cycles. | [54] |
Protocol I: Comprehensive Sperm Quality Evaluation
Defects in sperm function often occur despite normal conventional parameters. This protocol focuses on advanced functional and molecular assessments.
Sperm Functional Analysis
Principle: Move beyond concentration and motility to evaluate membrane integrity and DNA fragmentation, which are critical for successful fertilization and embryo development [55].
Materials:
- Sperm sample (from cauda epididymis or vas deferens)
- Phosphate-Buffered Saline (PBS)
- Propidium Iodide (PI) or SYBR-14/PI live-dead stain (e.g., LIVE/DEAD Sperm Viability Kit)
- Sperm Chromatin Structure Assay (SCSA) buffer or TUNEL assay reagents
- Flow cytometer or fluorescent microscope
- Computer-Assisted Sperm Analyzer (CASA) system (if available)
Procedure:
- Sperm Preparation: Collect sperm by dissection of the cauda epididymis into pre-warmed fertilization medium (e.g., FERTIUP). Allow sperm to capacitate for 30-60 minutes [13].
- Motility and Concentration with CASA:
- Place a small aliquot of the capacitated sperm suspension on a specialized counting chamber.
- Analyze using the CASA system to obtain objective, quantitative data on total motility (%), progressive motility (%), and sperm concentration [55].
- Membrane Integrity Assay:
- Dilute sperm to a concentration of ~1-2 x 10^6 sperm/mL.
- Incubate with SYBR-14 and Propidium Iodide (PI) according to the manufacturer's protocol.
- Analyze by flow cytometry or fluorescence microscopy. Viable sperm with intact membranes stain green (SYBR-14), while non-viable sperm with compromised membranes stain red (PI) [55].
- DNA Fragmentation Assessment:
- SCSA: Treat sperm with a low-pH detergent solution, then stain with Acridine Orange. Sperm with abnormal chromatin will fluoresce red, while those with normal DNA fluoresce green. Analyze by flow cytometry [55].
- TUNEL Assay: Fix and permeabilize sperm. Label DNA strand breaks with fluorescently tagged nucleotides. The percentage of TUNEL-positive sperm indicates the level of DNA fragmentation [52].
Diagnosis of Sperm-Induced Oocyte Activation Deficiency
Principle: The sperm-specific protein Phospholipase C zeta (PLCζ) is crucial for triggering the calcium oscillations that activate the oocyte [53]. Mutations or absence of PLCζ are a documented cause of total fertilization failure (TFF).
Materials:
- Donor sperm sample (patient/experimental)
- Control sperm sample (with known good fertilization capacity)
- Mature (Metaphase II) mouse oocytes
- Microinjection system (for ICSI)
- Calcium-sensitive dye (e.g., Fluo-4 AM)
- Fluorescence microscope with time-lapse capability
Procedure: Mouse Oocyte Calcium Analysis (MOCA)
- Oocyte Collection: Collect MII oocytes from superovulated female mice.
- Sperm Preparation: Prepare sperm heads for injection from both the test and control samples.
- Microinjection and Loading: Perform ICSI to inject a single sperm head into a mouse oocyte. Pre-load oocytes with a calcium-sensitive dye.
- Calcium Imaging: Immediately place injected oocytes under a fluorescence microscope in a controlled environment chamber. Record fluorescence for at least 60-90 minutes.
- Analysis:
- Positive Control: Sperm with normal PLCζ function will induce repetitive, high-amplitude calcium transients.
- Test Sample: A lack of calcium oscillations, or the presence of only a single, low-amplitude peak, indicates a defect in the sperm's oocyte activation capacity, potentially due to a PLCζ abnormality [53].
Protocol II: Oocyte Maturity and Collection Optimization
The developmental competence of the oocyte is a fundamental determinant of fertilization success.
Critical Timing for Oocyte Collection
Principle: The post-mortem interval between animal euthanasia and oocyte collection is a critical but often overlooked variable. Prolonged intervals can lead to oocyte aging and zona pellucida hardening, drastically reducing fertilization rates [15].
Materials:
- Superovulated female mice (e.g., C57BL/6J)
- Dissection instruments (fine scissors, forceps)
- Hyaluronidase solution
- Pre-warmed fertilization medium (e.g., CARD MEDIUM, mHTF)
Procedure:
- Superovulation: Inject pre-pubertal female mice with 5 IU of PMS (e.g., between 2:00-6:00 pm), followed by 5 IU of hCG 48-52 hours later [13].
- Timed Euthanasia and Collection: Sacrifice females approximately 15-17 hours post-hCG. The following steps must be performed swiftly and precisely.
- Strict Workflow Adherence:
- Step 1 (Oviduct Extraction): Dissect and extract the oviducts within 1 minute of euthanasia [15].
- Step 2 (Oocyte Release): Puncture the ampulla and release the cumulus-oocyte complexes (COCs) into pre-warmed fertilization medium. This step must be completed within 3 minutes of euthanasia [15].
- Step 3 (Incubation): Transfer COCs to the incubator promptly. The entire collection-to-incubation process should be minimized.
The following workflow diagram summarizes this critical path to ensure oocyte viability.
Morphological Assessment of Oocyte Maturity
Principle: For intra-cytoplasmic sperm injection (ICSI), oocytes must be stripped of surrounding cumulus cells and assessed for nuclear maturity based on the presence of the first polar body [56] [54].
Materials:
- Cumulus-Oocyte Complexes (COCs)
- Hyaluronidase solution
- Handling pipette
- Sterile culture dishes with microdroplets of washing medium
- Inverted microscope
Procedure:
- Cumulus Cell Removal: Incubate COCs in a hyaluronidase solution for a brief period (e.g., 1-2 minutes). Gently pipette to dissociate the cumulus cells.
- Washing: Wash the denuded oocytes thoroughly through several drops of clean culture medium.
- Maturity Grading: Examine under a microscope. A mature oocyte (Metaphase II) is identified by the clear presence of a single polar body. Oocytes without a polar body (Metaphase I) or with a germinal vesicle (Prophase I) are considered immature and have low fertilization potential [56] [54].
Integrated IVF Protocol for Murine Models
This protocol integrates the above assessments into a robust, step-by-step IVF procedure for timed embryo production.
Reagents & Supplies:
- FERTIUP: Sperm pre-incubation and capacitation medium [13].
- CARD MEDIUM: A specialized fertilization medium. Note: Preparation may vary based on sperm source (fresh vs. frozen) [13].
- mHTF: A standard medium for oocyte incubation and washing post-insemination [13].
- Hormones: PMS and hCG for superovulation.
- Mineral Oil: For embryo-tested oil to cover culture drops.
- Culture Dishes: 60 mm culture dishes for preparing drops.
Procedure:
- Superovulation and Oocyte Collection: Follow the superovulation and strict timed collection protocol outlined in Section 4.1. Incubate collected COCs in the CARD MEDIUM dish for 30-60 minutes before insemination [13].
- Sperm Preparation:
- Fresh Sperm: Dissect cauda epididymides from a stud male. Puncture to release sperm into a 100 µL drop of FERTIUP under oil. Allow to capacitate for 60 minutes in the incubator [13].
- Frozen Sperm: Thaw cryopreserved sperm rapidly according to vendor/strain-specific protocols. Process and wash sperm, then resuspend in FERTIUP for a 30-minute pre-incubation [13].
- Insemination:
- Add a calculated volume (e.g., 3-10 µL) of the motile sperm suspension from the top of the FERTIUP drop to the fertilization dish containing the COCs in CARD MEDIUM [13].
- Return the dish to the incubator and co-incubate sperm and oocytes for approximately 3-6 hours.
- Washing and Fertilization Check:
- After 3 hours, wash oocytes thoroughly through several drops of mHTF to remove excess sperm and cellular debris [13].
- Culture overnight.
- The next morning, check for successful fertilization by observing the formation of two pronuclei (2PN)—one from the sperm and one from the oocyte [53]. Two-cell embryos can then be cryopreserved or transferred to recipient females.
The Scientist's Toolkit: Essential Reagents
Table 2: Key Reagents for Murine IVF and Quality Checks
| Reagent/Kit | Primary Function | Application in Protocol |
|---|---|---|
| FERTIUP Medium | Sperm capacitation and pre-incubation. | Used to prepare the sperm sample before introduction to oocytes [13]. |
| CARD MEDIUM | Specialized medium to support fertilization. | The medium in which sperm and oocytes are co-incubated for insemination [13]. |
| mHTF Medium | General embryo culture and washing. | Used for washing oocytes/embryos post-insemination and for subsequent culture [13]. |
| Hyaluronidase | Enzyme that degrades hyaluronic acid. | Used to remove cumulus cells from the cumulus-oocyte complex (COC) for ICSI or assessment [54]. |
| LIVE/DEAD Sperm Viability Kit | Fluorescent stains for membrane integrity. | Differentiates between live (intact membrane) and dead (compromised membrane) sperm cells [55]. |
| SCSA or TUNEL Assay Kits | To assess sperm DNA fragmentation. | Quantifies the percentage of sperm with damaged DNA, a parameter correlated with embryo development [55] [52]. |
Successful and reproducible murine IVF hinges on a systematic approach that rigorously checks both sperm and oocyte factors. By implementing the detailed protocols for sperm functional analysis (including oocyte activation capacity) and adhering to the critical timing for oocyte collection, researchers can effectively diagnose the root causes of low fertilization. This enables targeted troubleshooting—such as using assisted oocyte activation for PLCζ-deficient sperm or optimizing superovulation schedules—leading to more reliable production of timed research embryos.
Within the context of a broader thesis on in vitro fertilization protocols for timed embryo donor mice research, this application note provides a detailed guide for optimizing procedures across three critical mouse strains: C57BL/6J, BALB/c, and NSG. These strains represent fundamentally different immunological and physiological backgrounds that profoundly influence their responses to superovulation, in vitro fertilization (IVF), and embryo transfer protocols. Strain-specific responses are a crucial component of experimental reproducibility in genetic engineering and biomedical research [57]. The C57BL/6 strain exemplifies a Th1-polarized immunological phenotype, while BALB/c mice tend toward Th2 immune responses, and NSG mice represent a severely immunodeficient model lacking both adaptive and natural killer cell function [58] [59] [60]. These inherent differences necessitate tailored approaches to IVF and embryo transfer protocols to maximize yield and experimental success. This document provides evidence-based, strain-specific protocols to guide researchers in optimizing their reproductive technologies for timed embryo production.
Strain Characteristics and Experimental Applications
The selection of an appropriate mouse strain requires a thorough understanding of their distinct physiological and immunological characteristics. These inherent differences not only define their primary research applications but also dictate the optimal approach to colony management and experimental design.
Table 1: Fundamental Characteristics of Common Laboratory Mouse Strains
| Characteristic | C57BL/6 | BALB/c | NSG (NOD SCID gamma) |
|---|---|---|---|
| Coat Color | Black | Albino | Albino [59] |
| Immune Profile | Th1-polarized; High interferon production [58] [60] | Th2-polarized; Stronger humoral response [58] [60] | Severely immunodeficient; Lacks T, B, and NK cells [59] |
| Major Histocompatibility Complex (MHC) | H2b [58] | H2d [58] | Derived from NOD background [59] |
| Primary Research Applications | Preferred genetic background for most site-directed modifications and transgenesis; Diet-induced obesity models [58] | Oncology, immunology, and infectious disease research; Hybridoma and monoclonal antibody production [58] | Xenografting (human tissue/tumor), immunodeficiency studies, virological research [59] |
| Key Considerations | Low tumor incidence; Macrophages resistant to anthrax lethal factor [58] | Susceptible to Listeria monocytogenes and respiratory pathogens; Higher cancer rates with age [58] | Requires strict pathogen-free conditions; Short lifespan; No gross behavioral abnormalities [59] |
The immunological divergence between C57BL/6 and BALB/c mice extends to their baseline gut microbiota composition, which is shaped by their respective Th1 and Th2 biases [60]. The NSG mouse, built upon a NOD/SCID background with an added interleukin-2 receptor gamma chain mutation, is one of the most immunocompromised strains available [59]. This profound immunodeficiency makes it invaluable for human tissue engraftment studies but also necessitates meticulous husbandry to prevent opportunistic infections.
Strain-Specific Protocol Optimization
Superovulation and IVF Responses
Superovulation efficacy is highly strain-dependent, requiring tailored hormonal administration protocols. Research has demonstrated significant variation in fertilization rates based on both mouse strain and the timing intervals used for administering pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) [57].
Table 2: Strain-Specific Responses to Superovulation and IVF Protocols
| Strain | Optimal PMSG/hCG Interval (Hours) | Fertilization Rate (2-Cell Stage) | Cumulus Cell Removal Effect |
|---|---|---|---|
| C57BL/6 | 55 / 14.5 (Group 3) [57] | Lower than F1 hybrids; influenced by timing [57] | No adverse effect on fertilization rate [57] |
| BALB/c | Protocol must be empirically determined | Information not specified in search results | Information not specified in search results |
| F1 Hybrid (C57BL/6J x SJL/J) | 55 / 14.5 (Group 3) [57] | Highest among tested strains [57] | No adverse effect on fertilization rate [57] |
| ICR (Outbred) | 60 / 14.5 (Group 2) [57] | Intermediate; influenced by timing [57] | No adverse effect on fertilization rate [57] |
The data indicates that the treatment protocol producing the highest fertilization rate is contingent upon the mouse strain, underscoring the necessity for empirical optimization [57]. Furthermore, the removal of cumulus cells during the IVF procedure does not adversely affect fertilization rates across strains, providing flexibility in experimental design [57].
Embryo Transfer and Foster Mother Selection
The choice of recipient foster mother significantly impacts the success of weaning germ-free pups derived via cesarean section. Recent findings challenge conventional wisdom regarding maternal care capabilities across different strains.
Table 3: Foster Mother Efficiency in Germ-Free Pup Weaning
| Foster Strain | Weaning Success Rate | Maternal Care Characteristics |
|---|---|---|
| BALB/c | Superior [5] | Exhibits superior nursing and weaning success [5] |
| NSG | Superior [5] | Exhibits superior nursing and weaning success [5] |
| KM (Outbred) | Information not specified | Information not specified |
| C57BL/6J | Lowest [5] | Lowest weaning rate, contrary to SPF maternal care data [5] |
Notably, the poor performance of germ-free C57BL/6J mice as foster mothers occurs in "stark contrast to findings on maternal care in SPF C57BL/6J foster mothers," highlighting a critical difference between specific pathogen-free (SPF) and germ-free (GF) environments or physiological states [5]. This finding is essential for planning rederivation projects and establishing germ-free lines.
Figure 1: A workflow for optimizing reproductive protocols across mouse strains, covering superovulation, IVF, and foster mother selection.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Reagents for Mouse IVF and Strain-Specific Optimization
| Reagent / Material | Function / Application | Strain-Specific Notes |
|---|---|---|
| Pregnant Mare Serum Gonadotropin (PMSG) | Mimics Follicle-Stimulating Hormone (FSH) to stimulate follicular development [57] | Timing relative to hCG is strain-dependent (e.g., 55h for C57BL/6, 48-60h range for others) [57] |
| Human Chorionic Gonadotropin (hCG) | Mimics Luteinizing Hormone (LH) to trigger ovulation [57] | Oocyte collection interval post-hCG is critical (e.g., 14.5h for C57BL/6) [57] |
| Embryo-Tested Water and Oil | Provides a stable, non-toxic medium for IVF and embryo culture | Quality is critical for all strains; no specific strain differences |
| Hormone Replacement Therapy (HRT) Reagents | Prepares the endometrium for embryo transfer in synchronized cycles [61] | Standard protocols may apply; no specific strain differences indicated |
| Clidox-S Disinfectant | Chlorine dioxide-based sterilant for germ-free isolators and surgical instruments [5] | Essential for immunodeficient NSG mice and germ-free colony maintenance [59] [5] |
The successful generation of timed embryo donor mice requires a sophisticated, strain-specific approach that accounts for profound differences in reproductive physiology, immunology, and maternal behavior. Key findings indicate that superovulation protocols must be empirically optimized for each genetic background, with C57BL/6 mice responding optimally to a 55-hour PMSG/hCG interval. Furthermore, the selection of foster mothers for germ-free pup derivation is critical, with BALB/c and NSG strains demonstrating superior weaning success compared to C57BL/6J. By integrating these evidence-based protocols for superovulation, IVF, and embryo transfer, researchers can significantly enhance the efficiency and reproducibility of their studies involving genetically engineered mouse models.
In vitro fertilization (IVF) for timed embryo donor mouse research relies critically on the precise preparation of sperm to achieve high fertilization rates and viable embryos. A key factor for successful IVF is sperm capacitation, a series of biochemical and functional changes that sperm undergo within the female reproductive tract to acquire the ability to fertilize an oocyte. Standard sperm preparation methods primarily select sperm based on motility and morphology, which are inadequate proxies for functional capacitation status [62] [63]. This often results in suboptimal embryo development rates, creating a demand for advanced techniques that better mimic the in vivo capacitation environment.
Novel approaches like the HyperSperm technique are designed to address this gap by optimizing the dynamic signaling pathways crucial for capacitation. This protocol details the application of such advanced sperm preparation methods within a research setting focused on producing timed embryos from donor mice, providing detailed methodologies, quantitative outcomes, and essential reagents to enhance experimental reproducibility.
Performance of HyperSperm vs. Standard Methods
The following table summarizes the key reproductive outcomes observed when using the HyperSperm technique compared to standard control preparation in a mouse model.
Table 1: Comparative Reproductive Outcomes of HyperSperm in a Mouse Model
| Parameter Measured | HyperSperm Result | Control Result | Statistical Significance (p-value) |
|---|---|---|---|
| Sperm Hyperactivation | Significantly Increased | Baseline | < 0.05 |
| Fertilization Rate (2-cell embryos) | Significantly Increased | Baseline | < 0.05 |
| Blastocyst Development Rate | Significantly Increased | Baseline | < 0.05 |
| Implantation Rate | Significantly Increased | Baseline | < 0.05 |
| Litter Size (Pups born per transferred blastocyst) | ~3.1 | ~0.9 | < 0.05 |
These results demonstrate that HyperSperm treatment significantly enhances multiple stages of the reproductive process, from initial sperm function to final litter size [64] [62] [63].
The composition of the capacitation medium itself is a critical variable. A side-by-side comparison of four common media (FD, HTF, TYH, and TYH with HEPES) revealed significant differences in their ability to support key sperm functions, as shown in the table below.
Table 2: Impact of Capacitation Media on Mouse Sperm Functional Parameters
| Media | Total Motility (B6CF1 mice) | Hyperactivation (B6CF1 mice) | Progesterone-Induced Acrosome Reaction (C57BL/6 mice) | Fertilization Rate with ZP-intact eggs (C57BL/6 mice) |
|---|---|---|---|---|
| FD | High | High | Lower | Highest |
| HTF | High | High | Not Induced | High |
| TYH | Lower | Lower | Highest | Intermediate |
| TYH (HEPES) | Intermediate | Lower | Intermediate | Lower |
This data highlights that no single medium is superior for all parameters. FD and HTF generally supported better motility and hyperactivation, while TYH was more effective for the acrosome reaction in C57BL/6 mice [65]. This underscores the need for media selection based on the specific functional endpoint required.
Detailed Experimental Protocols
HyperSperm Preparation Technique
The HyperSperm protocol is designed to recapitulate the dynamic in vivo capacitation process through sequential incubation in media that activate specific signaling pathways.
- Workflow Overview:
Materials:
- Sperm collection buffer (e.g., modified Tyrode's solution).
- Pre-warmed HyperSperm media (Medium A, B, and C). The exact formulations are proprietary but are designed to sequentially modulate pH and ion concentrations to activate pathways involving channels like CatSper and SLO3 [62] [63].
- Incubator maintained at 37°C and 5% CO2.
- Timed, superovulated female mice for oocyte collection.
Step-by-Step Procedure:
- Sperm Collection: Euthanize a proven male mouse according to approved animal welfare protocols. Excise the cauda epididymides and place them in a drop of pre-warmed collection buffer. Gently puncture the epididymides to release the sperm.
- Initial Incubation: Allow the sperm to disperse for 5-10 minutes. Then, carefully transfer a diluted sperm suspension to pre-equilibrated Medium A. Incubate for 30-45 minutes at 37°C/5% CO2.
- Sequential Activation: Using a fine pipette, collect motile sperm from the swim-up layer and transfer them to Medium B. Incubate for an additional 30-60 minutes.
- Final Capacitation: Finally, transfer the sperm to Medium C for the final capacitation phase, lasting 45-60 minutes. This step is critical for inducing hyperactivation.
- Assessment and IVF: Post-incubation, assess sperm concentration, motility, and hyperactivation using Computer-Assisted Sperm Analysis (CASA). Use the prepared sperm for IVF with cumulus-oocyte complexes (COCs) in the same medium or a standard fertilization medium.
Safety and Quality Control: In human sperm samples, HyperSperm demonstrated a high safety profile, with no adverse effects on total motility, viability, acrosome integrity, or DNA fragmentation compared to controls [64] [62]. It is recommended to run these quality control assays when establishing the protocol in a new laboratory.
To systematically evaluate the effect of different capacitation media on sperm function, the following protocol can be used.
- Workflow Overview:
Materials:
- Capacitation media for comparison (e.g., FD, HTF, TYH).
- Progesterone stock solution (for acrosome reaction induction).
- Coomassie Brilliant Blue stain or FITC-PNA for acrosome status.
- Computer-Assisted Sperm Analysis (CASA) system.
- Superovulated oocytes for IVF assay.
Step-by-Step Procedure:
- Sperm Preparation and Distribution: Collect sperm as described in section 3.1. After initial dispersion, divide the sperm suspension equally into several tubes. Pellet and resuspend each pellet in a different pre-warmed test medium (FD, HTF, TYH, etc.).
- Capacitation Incubation: Incubate all samples for 60-90 minutes at 37°C/5% CO2 to allow for capacitation.
- Functional Parameter Analysis:
- Motility and Hyperactivation: At the end of capacitation, take a small aliquot from each sample for analysis using the CASA system to determine total motility, kinematic parameters (VCL, ALH), and the percentage of hyperactivated sperm.
- Acrosome Reaction (AR): Split each capacitated sample into two aliquots. Add progesterone to one aliquot to induce the AR and a vehicle control to the other. Incubate further, then fix and stain sperm to assess acrosome status. Calculate the percentage of reacted sperm.
- In Vitro Fertilization Assay: Use the remaining capacitated sperm from each medium to inseminate cumulus-oocyte complexes (COCs) or zona pellucida-intact eggs in the same medium. The fertilization rate is calculated the next day as the percentage of two-cell embryos [65].
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Reagents for Advanced Sperm Capacitation Research
| Reagent/Material | Function in Protocol | Examples & Notes |
|---|---|---|
| Capacitation Media | Provides a defined chemical environment to support biochemical changes during capacitation. | FD, HTF, TYH. Choice of medium significantly impacts outcomes (see Table 2) [65]. HyperSperm uses a proprietary sequential media system [63]. |
| Ion Channel Modulators | Research tools to study the role of specific signaling pathways in capacitation. | Compounds targeting CatSper, SLO3, and Hv1 channels. Critical for understanding the mechanism of hyperactivation [62] [63]. |
| Hormones | Used to induce superovulation in donor females and to stimulate the acrosome reaction in sperm. | PMSG/hCG for superovulation. Progesterone for inducing the physiological acrosome reaction in quality control assays [65]. |
| Computer-Assisted Sperm Analysis (CASA) | Provides objective, quantitative assessment of sperm concentration, motility, and kinematic parameters. | Essential for quantifying hyperactivation, a key endpoint of successful capacitation [64] [65]. |
| Viability & DNA Integrity Assays | Assesses the safety and quality of sperm post-preparation. | Viability stains (eosin/nigrosin). DNA fragmentation assays (SCD, TUNEL). HyperSperm showed no detrimental effects on these parameters [62] [66]. |
The implementation of advanced sperm preparation techniques, such as the HyperSperm protocol, offers a significant opportunity to improve the efficiency and reproducibility of timed embryo production in mouse models. The quantitative data presented confirms that optimizing the capacitation process leads to enhanced fertilization, embryo development, and live birth outcomes. Furthermore, the choice of capacitation medium is a critical experimental variable that must be carefully considered and reported.
These protocols provide a foundation for researchers to standardize and enhance their IVF workflows. The consistent application of these detailed methods will not only improve the yield of timed donor embryos but also contribute to more reliable and translatable research outcomes in reproductive biology and drug development. Future work should focus on further elucidating the molecular mechanisms behind these techniques and adapting them for use with genetically diverse or challenging mouse strains.
Within the context of developing a standardized in vitro fertilization (IVF) protocol for timed embryo donor mice, the optimization of embryo culture conditions is paramount. The preimplantation period is marked by significant epigenetic reprogramming, and suboptimal in vitro conditions can impair embryonic development, viability, and the validity of research data [67]. This application note provides detailed methodologies and data-driven insights into optimizing the core components of the embryo culture system—media composition, temperature, and gas control—for murine models in preclinical research.
Media Composition and Formulation
The culture medium serves as the foundational element for supporting embryonic development from the zygote to the blastocyst stage. Its composition directly influences key metabolic and epigenetic processes.
Key Media Types and Historical Evolution
Most modern embryo culture media can be traced back to simple salt solutions like Ringer's solution [68]. Two predominant philosophies have guided media development: the "let the embryo choose" approach, which provides a constant array of nutrients from which the embryo can self-select, and the "back to nature" approach, which aims to dynamically mimic the changing environment of the female reproductive tract [68]. For murine embryo culture, the Potassium Simplex Optimized Medium (KSOM),- particularly KSOM supplemented with amino acids (KSOMaa), is currently a widely used and effective standard [68]. Its development was driven by the need to overcome the "two-cell block," a developmental arrest phenomenon common in in vitro cultured mouse embryos [68]. In contrast, sequential media systems (e.g., G1/G2) are designed to mirror the distinct metabolic substrate concentrations found in the oviduct versus the uterus [69].
Table 1: Key Commercially Available and Reference Media for Mouse Embryo Culture
| Media Name | Type | Key Characteristics | Primary Application |
|---|---|---|---|
| KSOM/KSOMaa [68] | Single-step | Formulated via simplex optimization; contains amino acids; overcomes the two-cell block in mice. | General mouse preimplantation embryo culture. |
| M16 [68] [70] | Single-step | A classic commercialized medium; may lack amino acids and glutamine in basic form. | Mouse embryo culture (often requires supplementation). |
| CZB [68] | Single-step | Excludes glucose and supplements glutamine to overcome the two-cell block. | Mouse embryo culture. |
| Cook / Vitrolife [71] | Sequential | Commercial media designed for human ART; can modulate calcium oscillations in mouse oocytes. | Human ART; used in comparative animal studies. |
| mR1ECM [68] | Sequential | Modified from hamster embryo culture medium; optimized for lower osmolality. | Rat preimplantation embryo culture. |
Impact of Media on Embryonic Development and Phenotype
Evidence strongly indicates that culture media composition has both immediate and long-term consequences. A 2025 study demonstrated that even short-term exposure (4 hours) of mouse oocytes to different human ART media (Cook and Vitrolife) immediately after fertilization significantly shaped early calcium (Ca²⁺) oscillations compared to the control medium KSOM [71]. These media resulted in fewer oscillations, lower frequency, and reduced variability. Crucially, these early signaling differences correlated with long-term phenotypic changes: female offspring from oocytes cultured in the human ART media were heavier throughout growth and had larger adult organs compared to the KSOM group [71]. This underscores that optimizing culture media is critical not just for development to blastocyst, but for the long-term health of the resulting offspring.
Furthermore, a novel approach using media with reduced nutrient concentrations (50% of standard levels of carbohydrates, amino acids, and vitamins) has shown promise. This formulation supported blastocyst development and resulted in embryos with more inner cell mass (ICM) cells and higher ATP levels than those cultured in standard control medium [69]. However, this also altered metabolic activity, indicating that nutrient levels can strategically shift embryonic metabolism.
Protocol: Mouse Embryo Culture in KSOMaa
Objective: To support the in vitro development of one-cell mouse embryos to the blastocyst stage. Reagents:
- KSOMaa medium (e.g., MilliporeSigma)
- Embryo-tested mineral oil
- Penicillin-Streptomycin-Glutamine (PSG) solution (optional) [70]
- MEM Non-Essential Amino Acids (NEAA) solution (if using base KSOM or M16) [70]
Procedure:
- Preparation: Equilibrate KSOMaa medium in a culture dish under oil (e.g., 20 µL microdrops under 1.4 mL oil) in a CO₂ incubator (5-6.5% O₂, 7.5% CO₂, 37°C) for at least 4 hours prior to use [72] [69].
- Group Culture: Transfer in vivo- or in vitro-derived one-cell embryos into the pre-equilibrated microdrops. To ensure optimal development, culture embryos in groups (e.g., 10 embryos per 20 µL drop) rather than singly [73].
- Culture Duration: Maintain the culture for 96-112 hours without a medium change if using a single-step medium like KSOMaa.
- Developmental Assessment: Monitor and record the rates of cleavage (day 2), blastocyst formation (day 4-5), and hatching (day 5) using a stereomicroscope or time-lapse system.
Diagram 1: Workflow for mouse embryo culture in KSOMaa.
Temperature Optimization
While 37°C is the standard culture temperature, recent studies suggest that a physiological temperature gradient may better mimic the in vivo environment.
Experimental Data on Temperature Variation
A 2022 mouse study investigated the effects of circadian temperature variations, comparing a lower-temperature group (T1: 37°C day/35.5°C night) and a higher-temperature group (T2: 38.5°C day/37°C night) against a constant 37°C control [72].
Table 2: Effects of Temperature Variation on Mouse Embryo Development [72]
| Parameter | Control (Constant 37°C) | T1 (37°C/35.5°C) | T2 (38.5°C/37°C) |
|---|---|---|---|
| Cleavage Dynamics | Normal | "Slow" cleavage | Similar to control |
| Blastocyst Morphology | Good-quality | Poor-quality | Similar to control, no adverse effects |
| Apoptotic Gene Expression | Baseline | Higher expression of Apaf1 | Similar to control |
| Amino Acid Metabolism | Normal profile | Stressed profile | Similar to control |
The study concluded that the lower-temperature treatment (T1) consistently impaired embryo development, whereas the higher-temperature regimen (T2) showed no detrimental effects and was comparable to the constant 37°C control [72]. This indicates that a slight elevation in temperature during the active phase is well-tolerated by mouse embryos.
Protocol: Implementing a Circadian Temperature Regime
Objective: To evaluate the effect of a physiological temperature gradient on embryo development. Equipment:
- Two time-lapse incubators or one with programmable temperature settings.
Procedure:
- Group Allocation: Randomly assign zygotes to one of two groups: Control (constant 37°C) or Variable (38.5°C for 12 hours, 37°C for 12 hours).
- Incubation: Culture the embryos in their respective groups for the entire preimplantation period (96-112 hours).
- Data Collection: Use time-lapse imaging to annotate key morphokinetic events (t2: time to 2-cell, tSB: time to start of blastulation, etc.), normalized to pronuclear fading (tPNf) to minimize bias [72].
- Endpoint Analysis: At the blastocyst stage, assess development rates, blastocyst quality, and perform molecular analyses (e.g., gene expression of stress markers like Igf2 and Apaf1) [72].
Gas Control and Physical Culture Parameters
The gaseous environment and physical culture setup are critical for minimizing cellular stress and supporting normal metabolic function.
Oxygen Tension
Atmospheric oxygen tension (~20%) is supra-physiological for preimplantation embryos, which develop in the oviduct and uterus at an oxygen tension of approximately 2-8% [67]. Culturing embryos under reduced oxygen tension (5-6.5%) is a well-established practice to reduce oxidative stress and improve embryo viability and development across multiple species [69]. Furthermore, the interaction between culture media and oxygen concentration is significant; the composition of the medium can influence how embryos respond to different oxygen levels [74].
Group Culture and Volume
The "group culture effect" is a well-documented phenomenon where embryos cultured in groups develop more efficiently than those cultured alone [73]. This is thought to be due to the beneficial effects of autocrine and paracrine factors secreted by the embryos themselves. Studies show that singly cultured embryos have significantly lower blastocyst development rates compared to those cultured in groups of 10 [73]. This requirement for group culture can be leveraged in experimental design by ensuring a consistent and adequate number of embryos per culture droplet (e.g., 10 embryos per 20 µL drop) [73]. Recent advances in microfluidics allow for the culture of small groups of embryos in defined, sub-microliter volumes (e.g., 100 nL), which can maintain critical concentrations of secreted factors and achieve high blastocyst development rates [73].
Diagram 2: Impact of oxygen tension and group culture on embryo development.
The Scientist's Toolkit: Essential Reagents and Equipment
Table 3: Key Research Reagent Solutions and Equipment
| Item | Function/Description | Example/Note |
|---|---|---|
| KSOMaa Medium | A chemically defined, single-step medium optimized for mouse preimplantation embryo culture. | Consider supplementing base M16 with MEM NEAA to improve blastocyst formation rates [70]. |
| Amino Acid Supplements | Provide building blocks for protein synthesis and can act as antioxidants and osmolytes. | |
| Embryo-Tested Mineral Oil | Overlays culture microdrops to prevent evaporation and maintain medium pH and osmolality. | Must be quality-tested using a mouse embryo assay [69]. |
| Portable Incubators | Maintain stable temperature and gas during short-term embryo handling outside the main incubator. | MIRI M multiroom incubator reduces environmental exposure [75]. |
| Time-Lapse Incubator | Allows continuous, non-invasive monitoring of embryo development for morphokinetic analysis. | EmbryoScope system [72]. |
| Low-Oxygen Incubator | Maintains a physiological oxygen tension (5-6.5%) to reduce oxidative stress on embryos. | Crucial for improving embryo quality and developmental potential. |
Optimizing embryo culture conditions for timed donor mice requires a holistic and evidence-based approach. Key recommendations include: the use of a well-formulated medium like KSOMaa under physiological low oxygen tension; the maintenance of stable, physiologically relevant temperature conditions, with evidence supporting tolerance to slight warming; and the practice of group culture to leverage beneficial embryo-to-embryo signaling. Adherence to these optimized protocols ensures the production of high-quality, developmentally competent embryos, thereby enhancing the reliability and reproducibility of data generated in preclinical research and drug development.
Validating Embryo Quality and Comparing Efficiency Across Strains and Techniques
Within the framework of developing a standardized in vitro fertilization (IVF) protocol for timed embryo donor mice, establishing clear and achievable benchmarks is fundamental for assessing laboratory proficiency and experimental success. This document outlines expected quantitative outcomes for key developmental stages—from fertilization to blastocyst formation—based on current research. Furthermore, it provides detailed methodologies and technical considerations to assist researchers in achieving these benchmarks, ensuring the consistent production of high-quality embryos for drug development and basic research.
Established Quantitative Benchmarks
Data compiled from recent studies provide concrete expectations for success in mouse IVF protocols. The tables below summarize key benchmarks for fertilization and embryonic development.
Table 1: Expected Fertilization and Early Development Rates in Mice
| Developmental Stage | Expected Rate | Key Influencing Factors |
|---|---|---|
| Fertilization (2-Cell Embryos) | ~60% (Post-Ovulation Mating) [76] | Timing of mating relative to ovulation; sperm quality and function. |
| Blastocyst Formation | Significant improvement with optimized sperm prep (p < 0.05) [62] | Sperm preparation method; culture conditions; protein stability and cell cycle duration [77]. |
| Usable Blastocyst Rate (Human - Reference) | 43.8% (Control) vs. 67.9% (Optimized Sperm Prep) [62] | Embryo quality assessment criteria; culture media supplements. |
Table 2: Developmental Timing and Implantation Potential in Mice
| Parameter | Typical Outcome | Notes |
|---|---|---|
| In Vivo Implantation Sites | Significantly higher with optimized sperm-derived embryos (p < 0.05) [62] | Indicator of embryo viability and uterine receptivity. |
| Live Pup Birth (from transferred blastocysts) | 3.1 ± 1.7 (Optimized) vs. 0.9 ± 1.2 (Control) [62] | Ultimate measure of protocol success and embryo health. |
| Interphase Duration (Blastocyst) | ~11-12 hours [78] | Slower cell cycle tempo compared to humans may influence development speed [77]. |
Detailed Experimental Protocols
Protocol 1: Synchronized Mating for Improved In Vivo Fertilization
This protocol is designed to maximize the yield of fertilized oocytes from timed donor mice by precisely coordinating copulation with ovulation.
Principle: Synchronizing the timing of mating with the onset of ovulation significantly increases the number of sperm reaching the ampulla, thereby improving fertilization efficiency, especially in superovulated females [76].
Workflow Diagram: In Vivo Fertilization Protocol
Materials & Reagents:
- IASe: A mixture of inhibin antiserum and equine chorionic gonadotropin (eCG) used for ultrasuperovulation to yield a high number of oocytes (e.g., CARD HyperOva) [76].
- hCG (Human Chorionic Gonadotropin): Used to trigger final oocyte maturation and ovulation [76].
- KSOM Medium: Potassium Simplex Optimized Medium, used for flushing and culturing embryos [76].
Procedure:
- Ultrasuperovulation: Administer an intraperitoneal injection of IASe (e.g., 0.1 mL IAS + 3.75 IU eCG) to 4-week-old female C57BL/6 mice at 18:00 [76].
- Ovulation Trigger: Approximately 48 hours after IASe injection, administer an intraperitoneal injection of 7.5 IU hCG at 18:00 [76].
- Timed Mating: Immediately after hCG injection, co-house the superovulated females with proven male breeders. Separate them into one of three timed groups:
- Pre-Ovulation: Co-housing for 0-10 hours post-hCG.
- During Ovulation: Co-housing for 10-15 hours post-hCG.
- Post-Ovulation: Co-housing for 15-19 hours post-hCG [76].
- Confirmation of Mating: Check female mice for the presence of a vaginal plug, which confirms copulation.
- Embryo Collection: Sacrifice the female mice 43-45 hours post-hCG injection. Flush the two-cell embryos from the oviducts using KSOM medium and count them [76].
Protocol 2: Optimized Sperm Preparation for Enhanced IVF Outcomes (HyperSperm)
This protocol describes a novel sperm preparation technique designed to better mimic in vivo capacitation, thereby improving fertilization rates and subsequent embryo development.
Principle: The HyperSperm method uses sequential incubation in different media to promote key signaling pathways for sperm capacitation and hyperactivation, leading to enhanced sperm function and improved blastocyst development and implantation rates [62] [64].
Workflow Diagram: Optimized Sperm Preparation (HyperSperm)
Materials & Reagents:
- cTYH Medium: A modified Toyoda-Yokoyama-Hosi medium containing methyl-β-cyclodextrin and polyvinyl alcohol, used for sperm preincubation [76].
- mHTF Medium: Modified Human Tubal Fluid, used as the fertilization medium during IVF [76].
- HYPERSPERM SOLUTIONS: Proprietary sequential media designed to dynamically mimic the female tract environment and promote sperm capacitation [62].
Procedure:
- Sperm Collection: Sacrifice a male mouse and collect sperm from the cauda epididymis into a drop of cTYH medium under liquid paraffin [76].
- HyperSperm Treatment: Incubate the sperm suspension using the sequential HyperSperm media protocol as described by the manufacturer. This typically involves:
- A first incubation step to activate initial signaling pathways.
- A second incubation step in a different medium to promote hyperactivation [62].
- Preincubation: Incubate the prepared sperm at 37°C in 5% CO₂ for 60 minutes to allow for capacitation [76].
- IVF Insemination: Add a 3 µL aliquot of the preincubated sperm suspension to a 200 µL drop of mHTF containing cumulus-oocyte complexes (COCs). Co-incubate for several hours to allow fertilization [76].
- Wash and Culture: Approximately 3 hours post-insemination, wash the oocytes to remove sperm and place them in fresh culture medium for subsequent development [76].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Mouse IVF and Embryo Culture
| Reagent / Solution | Function | Application Notes |
|---|---|---|
| IASe (e.g., CARD HyperOva) | Induces ultrasuperovulation, yielding a high number of oocytes per donor. | Effective in C57BL/6 strain; requires precise timing of hCG trigger [76]. |
| HyperSperm Media | Sequential media optimized to enhance sperm capacitation and hyperactivation. | Significantly improves blastocyst development and implantation rates vs. standard prep [62]. |
| KSOM Medium | Optimized culture medium for supporting preimplantation embryo development. | Used for embryo culture from the two-cell stage to blastocyst [76]. |
| mHTF Medium | Fertilization medium used during the insemination step of IVF. | Supports the interaction between sperm and oocytes [76]. |
| Autologous PRP | Supplement containing growth factors and cytokines to enhance embryo quality. | Adding 5% PRP to cleavage-stage medium improved usable embryo rates in poor-prognosis cases [79]. |
| H2B-mCherry mRNA | Reporter for live imaging of chromosomes and cell tracking in embryos. | Introduced via electroporation; allows monitoring of mitotic errors and cell fate [78]. |
Technical Notes for Enhanced Reproducibility
- Timing is Critical: The highest fertilization efficiency in superovulated mice is achieved when mating occurs during or after ovulation (10-19 hours post-hCG), yielding up to three times more embryos than pre-ovulatory mating [76].
- Monitor Developmental Tempo: Interspecies differences exist; mouse embryonic stem cells differentiate about 2.5 times faster than human cells. This is influenced by factors like protein stability and cell cycle duration, which should be considered in experimental timelines [77].
- Assess Blastocyst Quality: Use standardized grading criteria (e.g., Gardner and Schoolcraft system) focusing on blastocoel expansion, inner cell mass (ICM), and trophectoderm (TE) morphology. High-quality blastocysts are crucial for high implantation rates [79].
- Utilize Live Imaging: Optimized nuclear labeling and light-sheet microscopy allow for the non-invasive tracking of embryo development and the identification of de novo mitotic errors, such as lagging chromosomes and multipolar spindles, which can cause aneuploidy [78].
Within the context of timed embryo donor mouse research for in vitro fertilization (IVF), selecting a mouse strain with optimal reproductive performance is a critical determinant of experimental success. Efficiency in breeding colonies is measured by key metrics such as litter size, the rate of pregnancy establishment, and crucially, the weaning rate—the proportion of pups born that survive to the weaning stage. This application note provides a comparative analysis of common laboratory mouse strains, synthesizing quantitative data on these metrics to guide researchers in selecting the most efficient strain for generating embryos for IVF protocols. Furthermore, it details standardized experimental methodologies for evaluating strain performance, ensuring reproducible and efficient cohort generation for developmental and genetic engineering studies.
Strain Performance Data
The reproductive efficiency of laboratory mouse strains varies significantly due to their genetic background. The following tables consolidate key performance metrics from published studies to aid in strain selection.
Table 1: Comparative Litter Size and Reproductive Fecundity of Common Mouse Strains [80]
| Strain | Productive Matings | Litter Size | Number of Litters | Relative Fecundity |
|---|---|---|---|---|
| 129/SvJa | 75% | 5.9 | 4.1 | 18.1 |
| A/J | 65% | 6.3 | 2.9 | 11.9 |
| BALB/cJ | 47% | 5.2 | 3.8 | 9.3 |
| C57BL/6J | 84% | 7.0 | 4.0 | 23.5 |
| C3H/HeJ | 86% | 5.7 | 2.9 | 14.2 |
| DBA/2J | 75% | 5.4 | 3.9 | 15.8 |
| FVB/N | >90% | 9.5 | 4.8 | 41.0 |
| SJL/J | 72% | 6.0 | 3.1 | 13.4 |
Table 2: Postnatal Performance and Weaning Success in C57BL/6J Embryos Cotransferred with Different Strains [81] [5]
| Performance Metric | B6 & SJL/J Cotransfer | B6 & ICR Cotransfer | Notes |
|---|---|---|---|
| Pregnancy Rate | 100% | 100% | No adverse effect from either strain [81]. |
| Live Pups per Transferred Embryo | 41.8% (B6), 50.5% (SJL) | Data Incomplete | Similar numbers of live pups/embryos obtained [81]. |
| Postnatal Growth | Better | Standard | B6 pups showed better growth with SJL/J littermates [81]. |
| Weaning Rate of B6 pups (as GF Foster) | Low (Strain-specific) | Not Tested | C57BL/6J GF foster mothers had the lowest weaning rate [5]. |
| Weaning Rate of Foster Strain | Not Tested | Not Tested | BALB/c and NSG GF foster mothers showed superior weaning success [5]. |
Experimental Protocols
Protocol: Evaluating Strain Efficiency via Embryo Cotransfer
This protocol is designed to assess the impact of different carrier embryos on the maintenance of pregnancy and postnatal development of the strain of interest (e.g., C57BL/6J) [81].
- Animal Preparation: Use sexually mature mice (e.g., 8-16 week old ICR females) as embryo recipients. Maintain all animals under specific pathogen-free conditions with a 12-hour light/dark cycle.
- Embryo Production: Generate 2-cell stage embryos via in vitro fertilization (IVF) from the strains of interest (e.g., C57BL/6J) and the carrier strains (e.g., SJL/J or ICR). Cryopreserve and thaw embryos according to established methods prior to transfer [81] [5].
- Embryo Transfer: Transfer 6-7 embryos of the strain of interest and 6-7 carrier embryos into each oviduct of a 0.5 days post coitum (dpc) pseudopregnant recipient mouse. The total number of embryos transferred per recipient should be 27-28 [81].
- Pregnancy and Birth Monitoring: Allow recipients to carry pregnancies to term. Record the pregnancy rate and the number of live pups born for each strain in the litter.
- Postnatal Growth Assessment: On postnatal day 1 (P1), adjust the litter size to a standardized number (e.g., 3 pups per strain). Weigh each pup on P1, P7, and P21 to monitor postnatal growth [81].
- Weaning Rate Calculation: The weaning rate is calculated as the number of pups surviving to P21 divided by the number of pups present at P1 after litter standardization.
Protocol: Superovulation and IVF for Timed Embryo Production
This protocol outlines the process for obtaining a timed cohort of embryos from donor mice, which is critical for IVF and embryo transfer experiments [57].
- Superovulation: Inject pre-pubertal (e.g., 4-week-old) or young adult (e.g., 8-week-old) female mice intraperitoneally with pregnant mare serum gonadotropin (PMSG) to stimulate follicular development, followed by human chorionic gonadotropin (hCG) 46-48 hours later to induce ovulation.
- Sperm Collection: Collect sperm from the cauda epididymis of a sexually mature male (e.g., 12-15 weeks old) into a pre-warmed fertilization medium.
- In Vitro Fertilization: Place capacitated sperm droplets under oil in a culture dish. Add cumulus-oocyte-complexes (COCs) collected from the superovulated females into the sperm droplets and co-incubate for several hours.
- Embryo Culture: The following day, assess oocytes for successful fertilization by observing the formation of two pronuclei. Wash and culture the resulting zygotes in fresh medium. Fertilization rates are determined by the development to the 2-cell stage after overnight culture [57].
- Strain-Specific Optimization: Note that the optimal timing for PMSG/hCG administration and oocyte collection can vary significantly between mouse strains and must be optimized for maximum yield [57].
The Scientist's Toolkit
Table 3: Essential Research Reagents for Mouse IVF and Strain Efficiency Studies
| Reagent / Material | Function in the Protocol |
|---|---|
| Pregnant Mare Serum Gonadotropin (PMSG) | A hormone analog used to stimulate the synchronous development of multiple ovarian follicles in donor females (superovulation) [57]. |
| Human Chorionic Gonadotropin (hCG) | A hormone analog administered after PMSG to trigger final oocyte maturation and ovulation, allowing for timed collection of oocytes [57]. |
| Fertilization Medium | A specialized culture medium designed to support sperm capacitation, the acrosome reaction, and the fusion of sperm and oocyte during in vitro fertilization. |
| Embryo Culture Medium | A sequential series of specialized media that provide the necessary nutrients and conditions to support the development of zygotes into blastocysts in vitro. |
| Cryptoreprotective Agents (e.g., Glycerol, DMSO) | Chemicals used to protect embryos from ice crystal formation and osmotic damage during the freeze-thaw process for long-term storage [81]. |
| Pseudopregnant Recipient Mice | Female mice mated with vasectomized males; they cannot produce embryos but their reproductive tract is hormonally primed to receive and support transferred embryos to term [81]. |
Workflow and Signaling Diagrams
Strain Efficiency Workflow
Strain Selection Logic
The accurate assessment of embryo viability is a cornerstone of successful in vitro fertilization (IVF) protocols, particularly in genetically engineered mouse models where reproductive efficiency directly impacts research timelines and outcomes. The challenge of selecting embryos with the highest developmental potential is multifaceted, encompassing morphological, genetic, and metabolic dimensions. Recent advances in artificial intelligence (AI) assessment and molecular analysis of embryo secretome have provided powerful new tools for predicting implantation success and live birth outcomes. This application note provides a comprehensive framework for validating embryo viability through integrated assessment protocols, focusing specifically on timed embryo transfer in donor mice. By establishing standardized methodologies and quantitative benchmarks, researchers can significantly improve the efficiency of mouse model generation in reproductive and genetic research.
Performance Metrics of AI in Embryo Assessment
Table 1: Diagnostic performance of AI algorithms in predicting embryo implantation potential
| AI Model/System | Sensitivity | Specificity | Accuracy | AUC | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|---|---|
| Pooled AI Performance [82] | 0.69 | 0.62 | - | 0.70 | 1.84 | 0.50 |
| Life Whisperer [82] | - | - | 64.3% | - | - | - |
| FiTTE System [82] | - | - | 65.2% | 0.70 | - | - |
Developmental Outcomes in Euploid-Aneuploid Mosaic Embryos
Table 2: Cell lineage analysis in mouse embryos with induced chromosome mosaicism
| Developmental Stage | Embryo Group | Total Cell Count | Trophectoderm (TE) Cells | Epiblast (EPI) Cells | Primitive Endoderm (PE) Cells |
|---|---|---|---|---|---|
| Early Blastocyst (E3.5) [83] | Control | 40.9 | - | - | - |
| Reversine-treated | 37.6 | - | - | - | |
| Late Blastocyst (E4.5) [83] | Control | 95.9 | 78.2 | 9.4 | 8.3 |
| Reversine-treated | 72.3 | 60.0 | 5.9 | 6.4 | |
| Late Blastocyst (E4.5) [83] | Mad2 siRNA | 70.9 | - | 8.6 | - |
| Control siRNA | 83.0 | - | 11.4 | - |
Aneuploidy Induction and Developmental Impact
Table 3: Chromosome segregation errors and developmental outcomes following SAC inhibition
| Parameter | Control Embryos | Reversine-Treated (0.5 μM) | Post-Reversine Washout |
|---|---|---|---|
| Chromosome Segregation Errors [83] | 9.7% | 48.0% | 3.1% |
| Metaphase Length (minutes) [83] | 44.4 | 32.2 | 47.2 |
| Blastocyst Formation Rate [83] | 92% | 93% | - |
| Whole Chromosome Mis-segregation Rate [83] | 0% (0/37 cells) | 66.1% (39/59 cells) | - |
Experimental Protocols
Mouse Model of Chromosome Mosaicism
Principle: Induction of aneuploidy in pre-implantation embryos through reversible spindle assembly checkpoint (SAC) inhibition enables study of aneuploid cell fate and developmental competence of mosaic embryos.
Materials:
- Female mice (3-4 weeks old) for embryo collection
- M2 medium and KSOM embryo culture medium
- Reversine (Monopolar spindle 1-like 1 kinase inhibitor)
- Nocodazole (microtubule polymerization inhibitor)
- Histone H2B-GFP transgenic mice for live imaging
Procedure:
- Embryo Collection: Superovulate 3-4 week old female mice by intraperitoneal injection of 5 IU PMSG followed by 5 IU hCG 48 hours later. Mate with proven male mice immediately after hCG injection.
- Embryo Recovery: Collect zygotes from oviducts 20-24 hours post-hCG injection. Remove cumulus cells using hyaluronidase (300-500 IU/mL).
- SAC Inhibition: Culture embryos in M16 or KSOM medium at 37°C, 5% CO2 until 4-cell stage. Treat with 0.5 μM reversine in culture medium during the 4- to 8-cell division (approximately 12-24 hours).
- Recovery and Development: Wash embryos thoroughly to remove reversine. Continue culture in inhibitor-free KSOM medium until blastocyst stage (E4.5).
- Validation of Aneuploidy: Confirm chromosome missegregation rates through live imaging of H2B-GFP embryos or fixed-cell analysis using FISH for specific chromosomes (e.g., chromosomes 2, 11, 16).
- Developmental Assessment: Monitor blastocyst formation rates, cell number counts, and lineage specification by immunostaining for Cdx2 (TE), Nanog (EPI), and Sox17 (PE).
Quality Control:
- Include control embryos cultured in parallel without reversine treatment
- Validate SAC inhibition by co-incubation with 0.33 μM nocodazole - control embryos should arrest, while reversine-treated embryos should continue division
- Monitor metaphase duration as a quantitative measure of SAC inhibition; expected reduction from ~44 minutes to ~32 minutes
AI-Assisted Embryo Assessment Protocol
Principle: Artificial intelligence algorithms analyze morphological and morphokinetic parameters to objectively predict embryo implantation potential, reducing subjectivity in embryo selection.
Materials:
- Time-lapse incubation system (e.g., EmbryoScope)
- Standardized culture conditions and media
- AI analysis software (commercial or custom-built)
- Annotated dataset of embryo images with known outcomes
Procedure:
- Image Acquisition: Culture embryos in time-lapse system with image capture every 5-20 minutes throughout pre-implantation development.
- Feature Extraction: Algorithm automatically extracts morphokinetic parameters including:
- Timing of cleavage divisions (t2, t3, t4, etc.)
- Duration of cell cycles and synchronicity of divisions
- Blastocyst formation timing and expansion rate
- Cellular fragment distribution and dynamics
- Model Application: Input extracted parameters into validated AI model (e.g., Life Whisperer, FiTTE, or IVY).
- Viability Scoring: Algorithm generates quantitative viability score (0-1) or classification (high/medium/low potential) for each embryo.
- Transfer Priority: Rank embryos based on AI-derived scores for transfer decisions.
Validation:
- Compare AI predictions with embryologist assessments and eventual implantation outcomes
- Establish institution-specific performance metrics including sensitivity, specificity, and AUC
- Retrain models periodically with local outcome data to improve performance
Embryo Secretome Analysis Protocol
Principle: The secretome - molecules secreted by embryos into culture medium - provides non-invasive biomarkers of embryo viability, including genetic, metabolic, and proteomic information.
Materials:
- Individual culture medium droplets under oil for each embryo
- Mass spectrometry-grade reagents for proteomic analysis
- RNA extraction and sequencing kits for small non-coding RNA
- Digital PCR or next-generation sequencing platforms
Procedure:
- Conditioned Medium Collection: Culture embryos individually in 10-20 μL microdroplets under oil. At appropriate developmental stage (typically blastocyst), carefully remove 5-10 μL of spent medium without disturbing the embryo.
- Sample Preparation:
- For proteomic analysis: Concentrate samples using ultrafiltration, digest with trypsin, clean up with C18 columns
- For cell-free DNA analysis: Extract DNA using silica-membrane columns optimized for small fragments
- For small RNA analysis: Use specialized kits for microRNA and piRNA isolation
- Molecular Analysis:
- Perform LC-MS/MS for protein identification and quantification
- Conduct digital PCR or targeted sequencing for aneuploidy screening from cell-free DNA
- Implement small RNA sequencing for microRNA profiling
- Data Integration: Correlate secretome profiles with embryo morphology, developmental kinetics, and eventual implantation outcomes.
Biomarker Panels:
- Identify proteins associated with metabolic activity and stress response
- Quantify mitochondrial DNA release as potential viability marker
- Establish microRNA signatures correlated with ploidy status and developmental competence
Workflow Visualization
Embryo Viability Assessment Pathway
Aneuploid Cell Fate in Mosaic Embryos
Research Reagent Solutions
Table 4: Essential research reagents for embryo viability validation studies
| Reagent/Category | Specific Examples | Function/Application | Experimental Notes |
|---|---|---|---|
| SAC Inhibitors [83] | Reversine (0.5 μM), Mad2 siRNA | Induces chromosome segregation errors and aneuploidy for mosaic embryo models | Treatment during 4- to 8-cell division; reversible effects |
| Culture Media [7] | KSOM, M2 Medium, FHM | Supports embryo development and manipulation | Use FHM for embryo holding and washing during transfers |
| Lineage Markers [83] | Cdx2 (TE), Nanog (EPI), Sox17 (PE) | Immunostaining for lineage specification analysis | Critical for evaluating developmental effects of aneuploidy |
| Aneuploidy Detection [83] | FISH (Chr 2, 11, 16), Single-cell genome sequencing | Quantifies aneuploidy rates and types | Low-pass sequencing provides genome-wide view |
| AI Assessment Platforms [82] | Life Whisperer, FiTTE System, IVY | Objective embryo evaluation using morphokinetic data | Integrates image analysis with clinical data |
| Secretome Analysis [84] | Cell-free DNA, microRNA, Proteomic assays | Non-invasive embryo viability assessment | Requires specialized collection to avoid contamination |
| Hormonal Priming [7] | PMSG/hCG | Synchronizes donor and recipient reproductive cycles | Standard superovulation protocol for timed experiments |
Technical Considerations
Strain-Specific Variability
Reproductive performance in mouse models exhibits significant strain-dependent variability that must be considered in experimental design. The efficiency of aneuploidy induction via SAC inhibition may vary based on genetic background, potentially affecting the consistency of mosaic embryo models. Similarly, IVF success rates range from 5% to 90% across different mouse strains, necessitating pilot studies to establish strain-specific protocols [7]. This variability extends to response to hormonal priming for superovulation and embryo culture conditions, requiring optimization for each genetic background studied.
Analytical Validation
Robust validation of embryo assessment methodologies is essential for reliable results. AI algorithms require training on diverse, well-annotated datasets that include implantation and live birth outcomes to ensure generalizability across different laboratory conditions [82]. Secretome analysis demands stringent controls for background contamination and standardization of collection timing relative to developmental stage [84]. For chromosome mosaicism studies, comprehensive aneuploidy detection beyond a few chromosomal probes provides more accurate assessment of aneuploidy burden [83].
The integration of traditional morphological assessment with advanced technologies including AI-based morphokinetic analysis, secretome profiling, and chromosome screening provides a multidimensional approach to embryo viability validation. The mouse model of chromosome mosaicism demonstrates that aneuploid cells are progressively depleted during development, with lineage-specific fate determination - elimination by apoptosis in fetal lineages and proliferative impairment in placental lineages [83]. Critically, mosaic embryos maintain full developmental potential when sufficient euploid cells are preserved, highlighting the importance of comprehensive viability assessment beyond aneuploidy screening alone. These integrated protocols enable researchers to make data-driven decisions in embryo selection for timed transfers in donor mouse models, ultimately enhancing the efficiency of genetically engineered mouse production for biomedical research.
Comparative Analysis of Traditional vs. Fast Vitrification Protocols for Oocyte Cryopreservation
Oocyte cryopreservation has become a cornerstone of assisted reproductive technology (ART), with vitrification emerging as the gold standard method due to its superior outcomes compared to slow-freezing techniques [85] [86] [87]. Traditional vitrification protocols, while highly effective, are time-consuming and require prolonged oocyte exposure to potentially cytotoxic cryoprotectants [85] [88]. Recent advances have focused on developing fast vitrification and warming protocols that significantly reduce processing times while maintaining oocyte viability and developmental competence [86] [88]. This comparative analysis examines both traditional and novel rapid protocols within the context of preclinical research using animal models, particularly relevant for timed embryo donor mice research. The fundamental principle of vitrification involves ultra-rapid cooling to achieve a glass-like state that prevents intracellular ice crystal formation, which is lethal to cells [87]. Success in vitrification depends on the interplay of four key variables: cooling rate, warming rate, solution viscosity, and sample volume [87]. Recent evidence suggests that warming rate is potentially more critical than cooling rate for oocyte survival, as slow warming can permit deadly ice crystal formation through recrystallization [86] [87].
Comparative Performance Metrics
The transition from traditional slow-freezing methods to vitrification has markedly improved oocyte survival rates across species. A randomized controlled trial comparing vitrification methods to slow freezing for human cleavage-stage embryos demonstrated significantly higher post-warming survival rates with vitrification (87.6-89.4%) compared to slow freezing (63.8%) [89]. Similar improvements have been documented for oocytes, establishing vitrification as the preferred technique in most ART laboratories [85] [87].
Table 1: Comparative Outcomes of Traditional vs. Fast Vitrification Protocols in Animal Models
| Parameter | Traditional Vitrification | Fast Vitrification | Control (Fresh Oocytes) |
|---|---|---|---|
| Protocol Exposure Times | ES: 8-15 min; VS: ~1 min [86] | ES: 30 sec; VS: 30 sec [88] | N/A |
| Mouse Oocyte Survival Rate | 94.2% [88] | 97.2% [88] | 100% |
| Mouse Blastocyst Formation Rate | 83.4% [88] | 80.9% [88] | 86.4% [88] |
| Mouse Live Birth Rate | 47.8% [88] | 38.7% [88] | Not specified |
| Rabbit Blastocyst Formation Rate | 22.2% [88] | 28.6% [88] | Not specified |
| Human Oocyte Survival (Discarded Oocytes) | 94.1% [88] | 97.1% [88] | N/A |
Recent preclinical validation studies demonstrate that fast vitrification and warming protocols achieve comparable efficiency to traditional methods while significantly reducing processing time [88]. The fast protocol reduced exposure times to cryoprotectants without compromising oocyte integrity or developmental potential in mouse and rabbit models. Specifically, mouse oocytes subjected to the fast protocol showed excellent survival rates (97.2%) and blastocyst development (80.9%) comparable to traditional vitrification (94.2% survival, 83.4% blastocyst rate) [88]. Notably, embryo transfer outcomes in mice demonstrated no statistically significant adverse effects on implantation or full-term development, with live birth rates of 38.7% for fast vitrification compared to 47.8% for traditional methods [88].
Detailed Experimental Protocols
Traditional Vitrification Protocol for Mouse Oocytes
The traditional vitrification approach follows established methods with minor modifications for murine oocytes [89] [87].
Solutions and Reagents:
- Base medium: HEPES-buffered medium-199 or similar handling medium
- Equilibration Solution (ES): 7.5% (v/v) each of DMSO and ethylene glycol in base medium [89]
- Vitrification Solution (VS): 15% (v/v) each of DMSO and ethylene glycol, 0.5 M sucrose in base medium [89]
- Device: Cryotop or similar open carrier system
Procedure:
- Equilibration: Expose oocytes to ES at room temperature for 8-15 minutes [86].
- Vitrification: Transfer oocytes to VS for approximately 60 seconds at room temperature [86].
- Loading and Cooling: Load oocytes in minimal volume (<1μL) onto cryodevice and plunge directly into liquid nitrogen within 90 seconds of VS exposure [89].
- Storage: Transfer to long-term storage in liquid nitrogen tanks.
Traditional Warming Protocol:
- Preparation: Pre-warm warming solutions to 37°C.
- Initial Dilution: Transfer vitrified oocytes directly to 1.0 M sucrose solution for 1 minute at 37°C [86].
- Further Dilution: Move oocytes to 0.5 M sucrose solution for 3 minutes at 37°C.
- Washing: Transfer through two washes of base medium for 5 minutes each at 37°C.
- Culture Assessment: Transfer to culture medium and assess survival after 2 hours.
Fast Vitrification Protocol for Mouse Oocytes
The fast protocol substantially reduces exposure times while maintaining efficacy [88].
Solutions and Reagents:
- Base medium: HEPES-buffered medium-199 or similar handling medium
- Equilibration Solution (ES): 7.5% (v/v) each of DMSO and ethylene glycol in base medium
- Vitrification Solution (VS): 15% (v/v) each of DMSO and ethylene glycol, 0.5 M sucrose in base medium
- Device: Cryotop or similar open carrier system
Procedure:
- Equilibration: Expose oocytes to ES at room temperature for 30 seconds [88].
- Vitrification: Transfer oocytes to VS for 30 seconds at room temperature [88].
- Loading and Cooling: Load oocytes in minimal volume (<1μL) onto cryodevice and plunge directly into liquid nitrogen.
- Storage: Transfer to long-term storage in liquid nitrogen tanks.
Fast Warming Protocol:
- Preparation: Pre-warm warming solutions to 37°C.
- Dilution: Transfer vitrified oocytes directly to 0.5 M sucrose solution for 1 minute at 37°C [88].
- Washing: Move oocytes to washing solution for 1 minute at 37°C [88].
- Culture Assessment: Transfer to culture medium and assess survival after 2 hours.
Workflow Visualization
Figure 1: Comparative workflow of traditional versus fast vitrification protocols highlighting significant time reduction in fast methods.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagent Solutions for Oocyte Vitrification
| Reagent/Category | Specific Examples | Function & Importance |
|---|---|---|
| Permeating Cryoprotectants | Ethylene Glycol (EG), Dimethyl Sulfoxide (DMSO), Propylene Glycol (PROH) | Penetrate cell membrane, displace water, suppress ice formation [85] [87] |
| Non-Permeating Cryoprotectants | Sucrose, Trehalose | Create osmotic gradient, facilitate dehydration, prevent osmotic shock [85] [87] |
| Base Media | HEPES-buffered Medium-199, PBS with additives | Maintain pH stability during room temperature procedures [89] [87] |
| Vitrification Devices | Cryotop, Open Pulled Straw (OPS), Cryoloop | Enable ultra-rapid cooling rates (>10,000°C/min) via minimal volume [87] |
| Protein Supplement | Synthetic Serum Substitute, Dextran Serum Supplement | Stabilize membrane, prevent osmotic damage, reduce solution toxicity [89] |
Critical Considerations for Research Applications
Protocol Selection Factors
When implementing vitrification protocols for timed embryo donor mice research, several critical factors must be considered:
Oocyte Sensitivity: Oocytes present unique cryopreservation challenges due to their large size, sensitivity to chilling injury, and complex spindle structure [85] [86]. The meiotic spindle is particularly vulnerable to temperature fluctuations, and improper handling can lead to chromosomal alignment issues [86]. Fast protocols may mitigate this risk by reducing exposure to non-physiological conditions.
Cryoprotectant Toxicity: While essential for vitrification success, cryoprotectants pose concentration-dependent toxicity [85]. At 2.0 M concentration, cryoprotectants increase solution osmolarity to approximately 2,500 mOsm/kg, creating significant osmotic stress [85]. Fast protocols limit this exposure, potentially reducing cytotoxic and osmotic effects.
Training and Expertise: Both traditional and fast vitrification protocols are highly technique-dependent [87]. Consistent results require extensive training and strict quality control measures, including monitoring of individual operator outcomes and solution lot variations [87].
Application in Drug Development Research
For drug development professionals utilizing timed embryo donor mice models, vitrification protocols offer valuable applications:
Standardization: Cryopreservation enables batch testing of oocytes/embryos across multiple experiments, reducing biological variability in compound screening [88].
Genetic Preservation: Vitrification safeguards valuable genetic mouse lines, particularly important for models with reproductive challenges [88].
Toxicology Assessment: The sensitivity of vitrified-warmed oocytes to compound effects provides a robust model for reproductive toxicology studies [85] [88].
The comparative analysis of traditional versus fast vitrification protocols reveals that both approaches can effectively preserve oocyte viability and developmental competence in murine models. Fast protocols offer significant advantages in workflow efficiency, reducing total processing time from approximately 25-30 minutes to just 3 minutes while maintaining comparable survival, blastocyst formation, and live birth rates [88]. This enhanced efficiency makes fast vitrification particularly valuable in high-throughput research environments, including drug development studies using timed embryo donor mice. However, traditional protocols maintain their value as established, validated methods with extensive literature support. The choice between protocols should consider specific research objectives, technical expertise, and required throughput. As vitrification technology continues to evolve, further refinements in protocol efficiency and safety are anticipated, promising enhanced capabilities for reproductive research and biomedical applications.
Conclusion
A rigorously optimized and meticulously timed IVF protocol is fundamental to the efficient production of high-quality embryo donor mice, which are indispensable for reproducible research in genetics, drug development, and reproductive biology. Success hinges on integrating foundational knowledge of mouse reproductive cycles with a robust, step-by-step methodology, while proactively addressing strain-specific variations and common troubleshooting points. The adoption of advanced techniques, such as optimized sperm preparation and fast vitrification, can further enhance outcomes. Future directions should focus on standardizing protocols across laboratories, refining non-invasive embryo quality assessments, and exploring the long-term health of offspring derived from assisted reproductive technologies. By adhering to these principles, researchers can significantly improve the precision and efficiency of their animal model generation, thereby accelerating translational biomedical discoveries.
References
- 1. mRNA therapy restores sperm production and fertility in mice [https://www.eurekalert.org/news-releases/1101462]
- 2. Fertilin peptide decreases embryo resorption and improves ... [https://www.sciencedirect.com/science/article/pii/S2666335X25000618]
- 3. Rescue of male infertility by human PRSS55 in transgenic ... [https://www.nature.com/articles/s41598-025-09604-9]
- 4. In mice, fertility treatments linked to higher mutations than ... [https://www.jax.org/news-and-insights/2025/november/in-mice-fertility-treatments-linked-to-higher-mutations-than-natural-conceptions]
- 5. Optimized cesarean techniques, IVF use, and foster strain ... [https://www.nature.com/articles/s41598-025-05411-4]
- 6. A Strategic Analysis of C3 and C5 Humanized Mouse ... [https://www.cyagen.com/cyagen-lab-notes/c3-c5-mouse-model-drug-development]
- 7. Mouse Assisted Reproductive Technology (MART) Core [https://www.bu.edu/research/ethics-compliance/animal-subjects/mart/]
- 8. Estrous cycle [https://en.wikipedia.org/wiki/Estrous_cycle]
- 9. Estrus Cycle - an overview [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/estrus-cycle]
- 10. Successful use of HTF as a basal fertilization medium during ... [https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-023-06452-6]
- 11. Hormonal Control Of Reproduction [https://jackwestin.com/resources/mcat-content/reproductive-system/hormonal-control-of-reproduction]
- 12. The Normal Menstrual Cycle and the Control of Ovulation [https://www.ncbi.nlm.nih.gov/books/NBK279054/]
- 13. IVF CARD Protocol - Vanderbilt School of Medicine [https://medschool.vanderbilt.edu/vcmr/recovery-protocols/ivf-card-protocol/]
- 14. In Vitro Fertilization - Schulich School of Medicine & Dentistry [https://www.schulich.uwo.ca/lrtgt//about_us/protocols/in_vitro_fertilization.html]
- 15. Defined oocyte collection time is critical for reproducible in ... [https://www.sciencedirect.com/science/article/abs/pii/S0093691X20300066]
- 16. Matched Developmental Timing of Donor Cells with the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5995271/]
- 17. IVF Embryo Stages | IVF Embryo Development and Growth [https://www.ccrmivf.com/blog/embryo-stages-ivf/]
- 18. Timeline with images of embryo development in IVF [https://www.inviafertility.com/blog/blog/infertility/azzurrifan/timeline-embryo-development-ivf/]
- 19. IVF Embryo Development by Stages [https://www.cofertility.com/family-learn/ivf-embryo-development-stages]
- 20. Timed Pregnant Mice & Rats [https://www.taconic.com/resources/what-are-timed-pregnant-mice-rats]
- 21. 6 steps for setting up timed pregnant mice [https://www.jax.org/news-and-insights/jax-blog/2014/september/six-steps-for-setting-up-timed-pregnant-mice]
- 22. Dynamic revelation of early development in mouse embryo ... [https://www.sciencedirect.com/science/article/abs/pii/S1386142525007814]
- 23. Supplementation with oviductal EVs from the estrus ... [https://www.nature.com/articles/s41598-025-07195-z]
- 24. In Vitro Fertilization (IVF) [https://card.medic.kumamoto-u.ac.jp/card/english/sigen/manual/mouseivf.html]
- 25. Genetically Engineered Rodent Models Core Services | BCM [https://www.bcm.edu/research/atc-core-labs/genetically-engineered-rodent-models-core/services]
- 26. A simple and economic protocol for efficient in vitro ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8553151/]
- 27. Mouse Embryo Media [https://www.sigmaaldrich.com/US/en/products/cell-culture-and-analysis/cell-lines-and-specialty-cell-culture/mouse-embryo-media?srsltid=AfmBOorAJnMaY-Dui-wNOwQTEbgCKWF_LLMr8V_vHJdtASHEHNFhV5cL]
- 28. Cryopreservation Protocols for Genetically Engineered Mice [https://pmc.ncbi.nlm.nih.gov/articles/PMC8211118/]
- 29. Mouse female superovulation guideline - Stanford Medicine [https://med.stanford.edu/tktc/service/transgenesis-animal-services/transgenic-service/mouse-female-superovulation-guideline-.html]
- 30. Mouse Superovulation Protocol [https://ilexlife.com/blogs/news/mouse-superovulation-protocol?srsltid=AfmBOoqbW7QKxTg0y4lMR7QvxA6kpNuKi3SfzBvyR_YgF6mUjbl74XOv]
- 31. Superovulation Protocol [https://www.taconic.com/resources/superovulation-protocol]
- 32. Superovulation Strategies for 6 Commonly Used Mouse ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3148645/]
- 33. Isolation and in vitro Culture of Mouse Oocytes - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8376588/]
- 34. Collection of Semen [https://www.sart.org/patients/a-patients-guide-to-assisted-reproductive-technology/general-information/collection-of-semen/]
- 35. In Vitro Fertilization | Center for Mouse Genome Modification [https://health.uconn.edu/mouse-genome-modification/services/in-vitro-fertilization/]
- 36. Fresh versus frozen micro-TESE sperm and outcomes - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12112934/]
- 37. Sperm Retrieval Procedures [https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/sperm-retrieval-procedures]
- 38. Sperm preparation after freezing improves motile ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5845028/]
- 39. o-07 microfluidic sperm sorting on fresh and frozen ivf cycles [https://www.sciencedirect.com/science/article/abs/pii/S1472648324007235]
- 40. Sperm freezing [https://www.hfea.gov.uk/treatments/fertility-preservation/sperm-freezing/]
- 41. Fresh and Frozen Sperm for IUI and IVF Cycles [https://www.esco-medical.com/news/fresh-and-frozen-sperm-for-intrauterine-insemination-iui-and-in-vitro-fertilization-ivf-cycles]
- 42. Mouse embryo and oocyte collection and IVF [https://bio-protocol.org/exchange/minidetail?id=265723&type=30]
- 43. On-chip oocyte cumulus removal using vibration-induced flow [https://pubs.rsc.org/en/content/articlehtml/2025/lc/d5lc00414d]
- 44. Robotic Manipulation of Cumulus–Oocyte Complexes for ... [https://www.mdpi.com/2076-3417/14/18/8450]
- 45. Brief and long co-incubation of sperm and oocytes for in vitro ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10035113/]
- 46. Improved clinical outcomes with a modified warming protocol ... [https://ovarianresearch.biomedcentral.com/articles/10.1186/s13048-025-01776-2]
- 47. IVF Process Day by Day - In Vitro Fertilization Steps [https://www.ccrmivf.com/fertility/ivf-process/]
- 48. IVF Step-by-step | UR Medicine [https://www.urmc.rochester.edu/strong-fertility-center/services/infertility/infertility-treatment-options/ivf/ivf-step-by-step]
- 49. Transcervical Embryo Transfer in Mice - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4128559/]
- 50. Culture media affect sex after IVF treatment—a detailed ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11143141/]
- 51. C57BL/6J and B6129F1 Embryo Transfer [https://www.mdpi.com/2076-2615/10/8/1424]
- 52. Poor fertilization in IVF is usually due to sperm, not the egg [https://www.remembryo.com/poor-fertilization-in-ivf-is-usually-due-to-sperm-not-the-egg/]
- 53. Low Fertilization Rate with ICSI: Reasons - My IVF Answers [https://www.myivfanswers.com/video/failed-ivf-icsi/]
- 54. Total fertilization failure: is it the end of the story? - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4156939/]
- 55. Semen evaluation: methodological advancements in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8255896/]
- 56. Methods for Assessing Oocyte Quality: A Review of Literature [https://pmc.ncbi.nlm.nih.gov/articles/PMC9495944/]
- 57. In vitro fertilization in mice: Strain differences in response to ... [https://pubmed.ncbi.nlm.nih.gov/16728073/]
- 58. C57BL/6 vs BALB/c Mice: Key Differences for Researchers [https://www.cyagen.com/cyagen-lab-notes/balbc-vs-c57bl6-mouse]
- 59. NSG Mouse Strain Characteristics - Maze Engineers [https://maze.conductscience.com/nsg-mouse-strain/]
- 60. Host Immunity Influences the Composition of Murine Gut ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8965567/]
- 61. Effect of Timing by Endometrial Receptivity Testing vs ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9856480/]
- 62. Increased reproductive outcomes after optimized sperm ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12107353/]
- 63. Increased reproductive outcomes after optimized sperm ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2025.1596421/full]
- 64. Increased reproductive outcomes after optimized sperm ... [https://pubmed.ncbi.nlm.nih.gov/40433542/]
- 65. A side-by-side comparison of different capacitation media ... [https://www.nature.com/articles/s41598-024-65134-w]
- 66. A Narrative Review on the Sperm Selection Methods in ... [https://www.mdpi.com/2077-0383/14/4/1066]
- 67. Association Between Human Embryo Culture Conditions ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12298697/]
- 68. Evolution of Media Supporting the Development ... [https://www.mdpi.com/2079-7737/13/10/789]
- 69. A novel culture medium with reduced nutrient ... [https://www.nature.com/articles/s41598-020-66019-4]
- 70. Optimization of In Vitro Murine Embryo Culture Condition ... [https://doaj.org/article/415d891bf11743b18dd42604bdbac0b6]
- 71. Short-Term Exposure to Culture Media Used in Human ... [https://pubmed.ncbi.nlm.nih.gov/41245006/]
- 72. The effects of temperature variation treatments on ... [https://www.nature.com/articles/s41598-022-06158-y]
- 73. In Vitro Embryo Culture in Defined, Sub-microliter Volumes [https://pmc.ncbi.nlm.nih.gov/articles/PMC2678198/]
- 74. Composition of single-step media used for human embryo ... [https://www.sciencedirect.com/science/article/pii/S0015028217300419]
- 75. Elevating Embryo Culture Standards: The MIRI® M ... [https://www.esco-medical.com/news/elevating-embryo-culture-standards-the-miri-m-advantage]
- 76. Synchronization of the ovulation and copulation timings ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9901804/]
- 77. Species-specific developmental timing is associated with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7116327/]
- 78. Live imaging of late-stage preimplantation human embryos ... [https://www.nature.com/articles/s41587-025-02851-1]
- 79. The use of autologous platelet-rich plasma in embryo ... [https://www.nature.com/articles/s41598-025-07688-x]
- 80. 4 Considerations for Breeding Laboratory Mice [https://www.taconic.com/resources/easier-said-than-bred-4-considerations-for-breeding-laboratory-mice]
- 81. Comparison of SJL/J and ICR as Cotransferred Carrier Embryos [https://pmc.ncbi.nlm.nih.gov/articles/PMC4244286/]
- 82. Predicting pregnancy outcomes cycles: a systematic review and... in IVF [https://contraceptionmedicine.biomedcentral.com/articles/10.1186/s40834-025-00400-4]
- 83. of chromosome mosaicism... | Nature Communications Mouse model [https://www.nature.com/articles/ncomms11165?error=cookies_not_supported&code=a0501948-3c33-4abd-8449-30d8e800a931]
- 84. secretome in predicting Embryo quality and embryo treatment... IVF [https://pubmed.ncbi.nlm.nih.gov/40446645/]
- 85. Oocyte vitrification: advances, progress and future goals [https://pmc.ncbi.nlm.nih.gov/articles/PMC3969469/]
- 86. The new ice age: the promise and challenges of rapid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11621260/]
- 87. A review of best practices of rapid-cooling vitrification for ... [https://www.asrm.org/practice-guidance/practice-committee-documents/a-review-of-best-practices-of-rapid-cooling-vitrication-for-oocytes-and-embryos-a-committee-opinion-2021/]
- 88. Preclinical validation of fast oocyte vitrification and ... [https://pubmed.ncbi.nlm.nih.gov/40334080/]
- 89. A randomized controlled trial comparing two vitrification ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3933602/]