Research Articles
Troubleshooting High Background in Whole-Mount In Situ Hybridization of Regenerating Tissues: A Comprehensive Guide
Whole-mount in situ hybridization (WISH) is an indispensable technique for visualizing spatio-temporal gene expression patterns during the complex process of tissue regeneration.
Understanding and Controlling Nonspecific Probe Binding: Sources, Impacts, and Solutions for Molecular Assays
Nonspecific probe binding is a critical challenge that compromises the accuracy and reliability of hybridization-based techniques essential to genomics, diagnostics, and drug development.
Achieving High Signal-to-Noise Ratio in FISH: A Comprehensive Guide from Probe Design to Automated Analysis
This article provides a systematic framework for researchers and drug development professionals to maximize the signal-to-noise ratio (SNR) in Fluorescence in situ Hybridization (FISH) experiments.
Unmasking the Signal: A Comprehensive Guide to Background Staining Causes and Solutions in Whole-Mount In Situ Hybridization
This article provides a systematic analysis of the factors contributing to background staining in whole-mount in situ hybridization (WISH), a critical challenge for researchers and drug development professionals.
Solving High Background in Whole-Mount In Situ Hybridization: A Comprehensive Troubleshooting Guide for Researchers
High background staining is a common and frustrating challenge in whole-mount in situ hybridization (WISH) that can obscure genuine gene expression signals.
Conquering Background Noise: A Researcher's Guide to Optimizing Embryo In Situ Hybridization
Background noise and autofluorescence present significant challenges for achieving clear, reliable results in embryo in situ hybridization (ISH), impacting data accuracy in developmental biology, drug research, and diagnostics.
Optimizing HDR Efficiency in Zebrafish Embryos: Advanced Strategies for Precision Genome Engineering
This comprehensive review synthesizes current methodologies for enhancing homology-directed repair (HDR) rates in zebrafish knock-in experiments, addressing a critical bottleneck in precision genome editing.
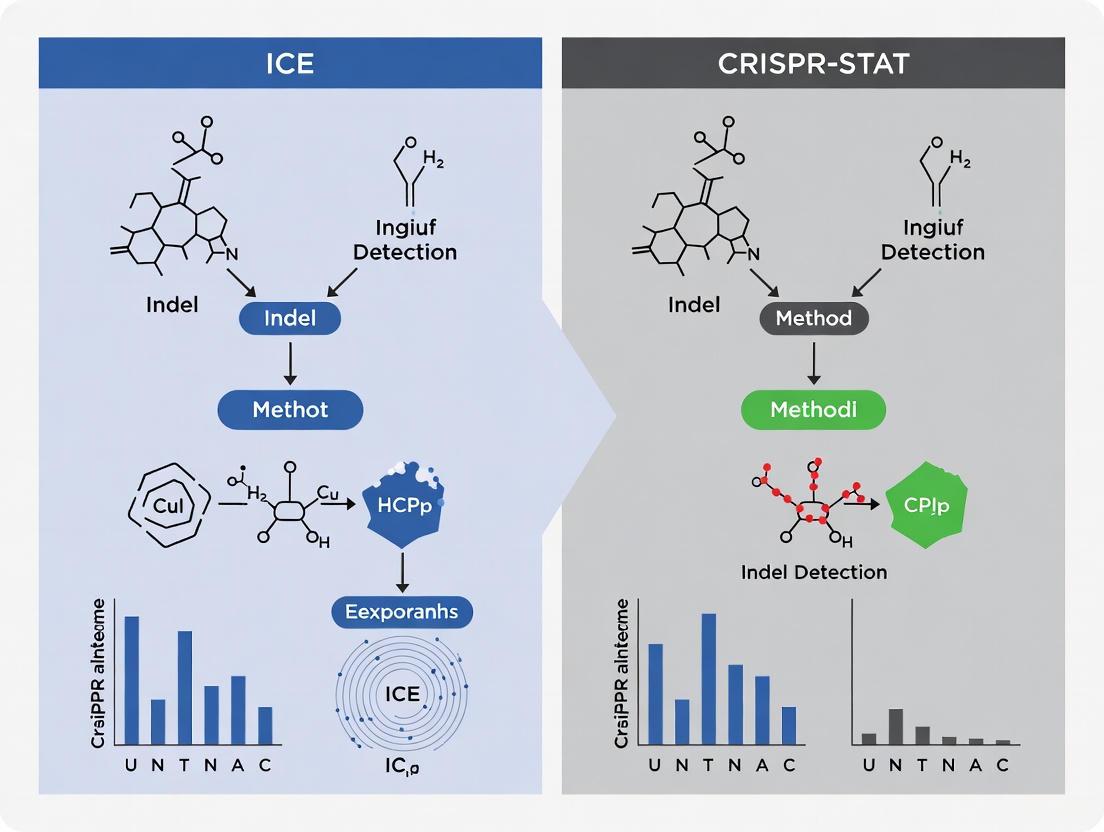
ICE vs. CRISPR-STAT: A Comparative Analysis of Indel Detection Methods for CRISPR Genome Editing
This article provides a comprehensive comparative analysis of two prominent indel detection methods, ICE (Inference of CRISPR Edits) and CRISPR-STAT (Somatic Tissue Activity Test).
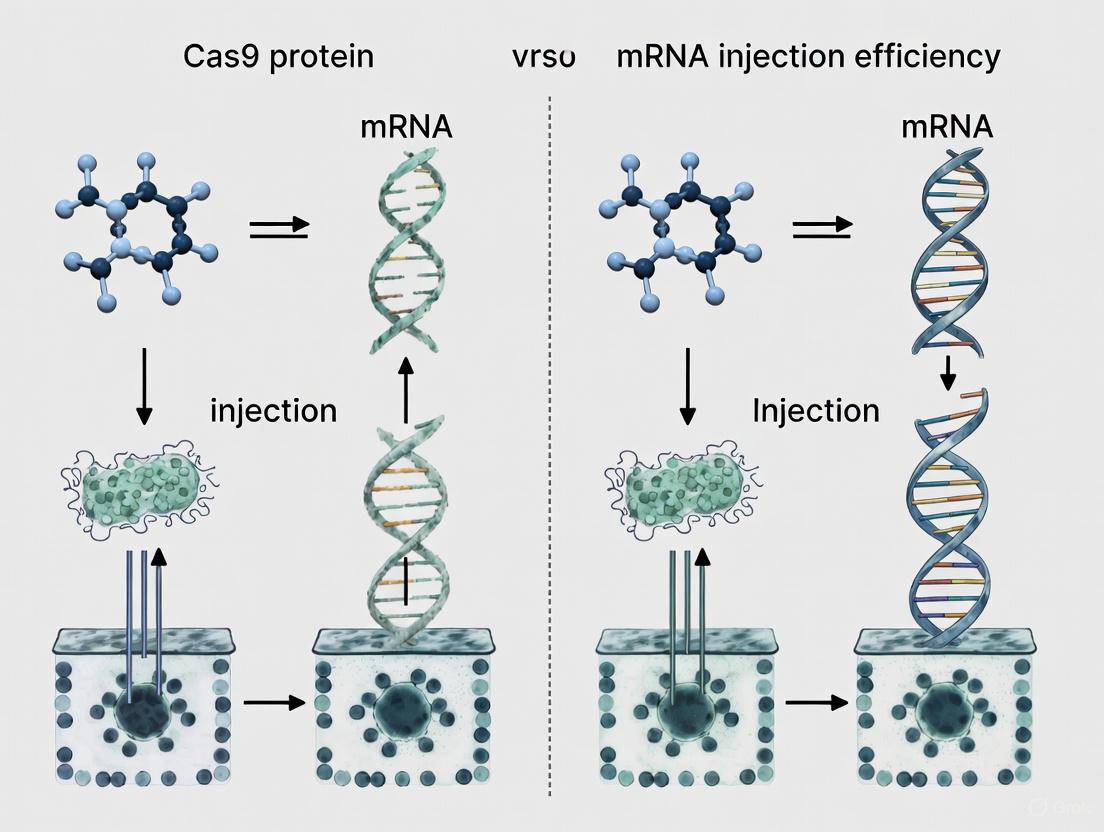
Cas9 Protein vs. mRNA Delivery: A Strategic Guide to Optimizing Editing Efficiency for Therapeutics
This article provides a comprehensive analysis for researchers and drug development professionals on the critical choice between delivering the CRISPR-Cas9 system as a pre-complexed protein (RNP) or as mRNA.
Multi-Locus Targeting with Synthetic gRNAs in Zebrafish: A Revolutionary F0 Knockout Strategy for Accelerated Biomedical Research
This article details the transformative methodology of multi-locus targeting using synthetic guide RNAs (gRNAs) in zebrafish, a technique that enables highly efficient, biallelic gene knockouts directly in the F0 generation.